Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Sep 28, 2025
In the highly regulated and technically demanding world of e-liquid manufacturing, precision is not a luxury—it’s a necessity. The foundation of this precision, particularly concerning your product’s most defining characteristic—its flavor—rests entirely upon a single, critical document: the Flavor Specification Sheet (Spec Sheet).
For flavorists, chemists, procurement managers, and e-liquid brand owners, the Spec Sheet is more than just a list of ingredients; it is the technical blueprint that dictates how a flavor concentrate must be handled, formulated, stored, and regulated. Misinterpreting this document can lead to batch inconsistencies, regulatory non-compliance, shelf-life issues, and ultimately, a failed product launch.
This comprehensive guide is designed to serve as an authoritative, technical walkthrough for e-liquid professionals, clarifying every essential section of a Flavor Spec Sheet. By understanding the granular detail within this document, you can ensure the quality, safety, and scalability of your e-liquid formulations.
Laboratory Technician Reviewing Data
Every spec sheet begins with the fundamental administrative details. While seemingly mundane, these identifiers are crucial for supply chain management, quality control (QC) tracking, and regulatory filing.
The Product Code (SKU) is the unique alphanumeric identifier for the exact flavor formulation. It ensures that the correct flavor is consistently ordered and received. Equally important is the Revision Number (or Version Date). Flavors can be adjusted over time for cost optimization, regulatory changes, or performance improvements.
The Legal Name is often the formal description used in regulatory submissions (e.g., “Strawberry Flavoring, Natural & Artificial”). The Common Name is the marketing or operational name (e.g., “Ripe Summer Strawberry”). Understanding both is necessary for internal inventory and external compliance labeling.
This section is paramount for logistics and inventory management. It details the flavor’s stability period—typically given in months (e.g., 12 or 24 months) from the manufacturing date, provided specific storage conditions are met.
This is the technical heart of the Spec Sheet, detailing the measurable characteristics that QC teams must verify upon receipt.
These are the simplest metrics for initial QC checks:
These are the non-negotiable quantitative data points:
This section reveals the composition and is vital for product safety, solubility, and understanding how the flavor will perform in the e-liquid base.
E-liquid flavors are concentrates that require a solvent to remain liquid and dispersible. Common carriers for e-liquid flavors include:
This is perhaps the single most scrutinized section in the modern e-liquid industry. Concerns over the inhalation risks of these diketones have led many manufacturers to require certified Diketone-Free flavors.
(Quote/Reference 1: Government/Regulatory Source)
“Regulatory bodies, such as the European Union through the Tobacco Products Directive (TPD), place significant restrictions or outright bans on the use of certain substances, including specific flavor compounds like Diacetyl, in e-liquids due to potential respiratory concerns.” (Source: A Relevant EU/FDA/Health Canada Regulatory Guideline or Fact Sheet on Flavoring Compounds in Vaping Products).
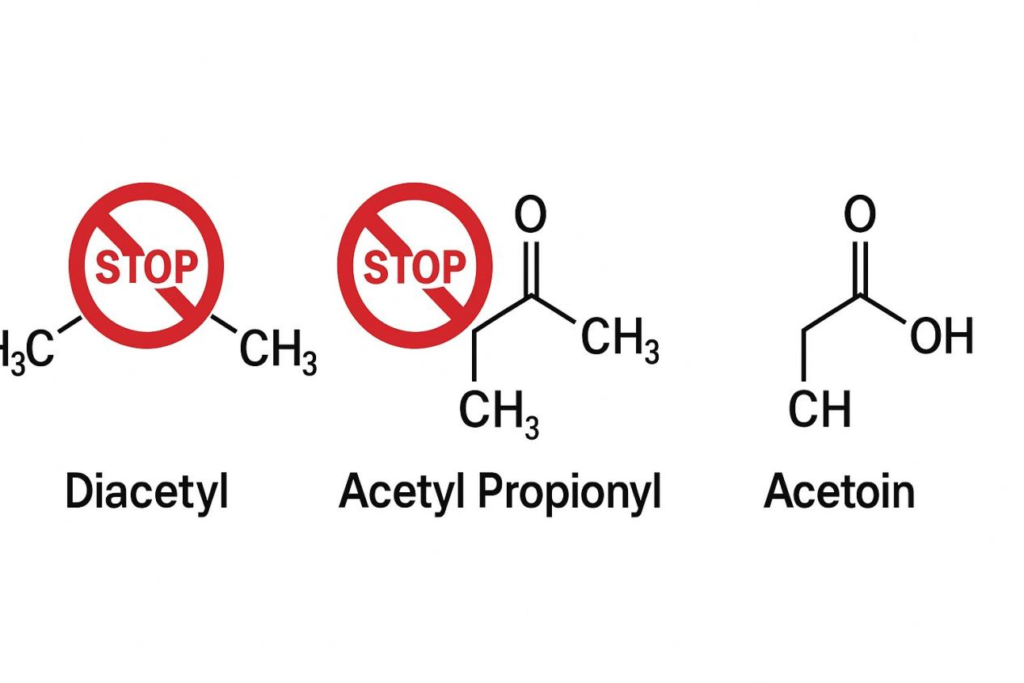
Chemical Structures of DA, AP, and Acetoin
The Spec Sheet acts as the primary document for legal compliance and consumer safety reporting.
This lists all components used in the flavor. For proprietary reasons, the flavor house may not list every single component but must list components above a certain threshold, or those that are known allergens.
(Quote/Reference 2: Industry Association/Research Report)
“The FEMA GRAS program is the longest-running and most comprehensive program for the safety evaluation of flavoring substances, although e-liquid manufacturers must still conduct specific inhalation risk assessments beyond GRAS status alone.” (Source: FEMA Official Website or an authoritative industry flavor research paper).
This is the flavor house’s expert recommendation for the optimal inclusion percentage in the finished e-liquid (e.g., 8% to 12% by weight). This range is crucial because it balances:
Every Spec Sheet is inherently tied to a comprehensive Safety Data Sheet (SDS) (formerly MSDS). The SDS contains 16 sections detailing:
For the flavor chemist and serious R&D professional, a high-quality Spec Sheet provides additional, non-mandatory information that aids in sophisticated formulation.
Advanced sheets may detail the purity of a flavor’s key characterizing components or specify the maximum allowable limits for impurities (e.g., heavy metals, residual solvents). This is often achieved through high-performance liquid chromatography (HPLC) or gas chromatography/mass spectrometry (GC/MS) analysis.
(Quote/Reference 3: Professional Journal/Education Institution)
“GC/MS is the gold standard for analyzing the volatile organic compounds (VOCs) in a flavor concentrate, allowing for the precise quantification of trace elements and potential contaminants, which is essential for ensuring batch-to-batch consistency and meeting international quality standards.” (Source: A peer-reviewed chemistry journal article or an accredited university’s analytical chemistry resource).
Some flavor houses provide a Suggested Steep Time (e.g., 7 days) and Temperature Testing Data. This information guides the manufacturer on how long the flavor needs to fully integrate with the PG/VG base to achieve its optimal profile, helping to eliminate “tasting young” or “off-flavor” complaints.
The Spec Sheet is not an island; it is part of a quality documentation system. Understanding this hierarchy is essential for successful auditing and compliance.
The Spec Sheet establishes the required or target specifications. The Certificate of Analysis (CoA) is the document that proves the actual batch of flavor you received meets those specifications.
High-quality flavor houses utilize a strict Change Control process. If any ingredient, percentage, or process changes, a new Revision Number is issued, and a Change Notification Letter is sent. This process is critical for regulatory submissions, which must reference the exact formulation and version used.
(Quote/Reference 4: Wikipedia/Reputable News Media/Business Source)
“In regulated industries like pharmaceuticals and, increasingly, e-liquids, Change Control is a formal process used to ensure that all proposed changes to a product or system are properly documented, reviewed, and approved before implementation. Failure to adhere to change control procedures can invalidate regulatory approvals and lead to product recalls.” (Source: Wikipedia entry on Change Control or a relevant reputable business/news article discussing GxP or Quality Management Systems).
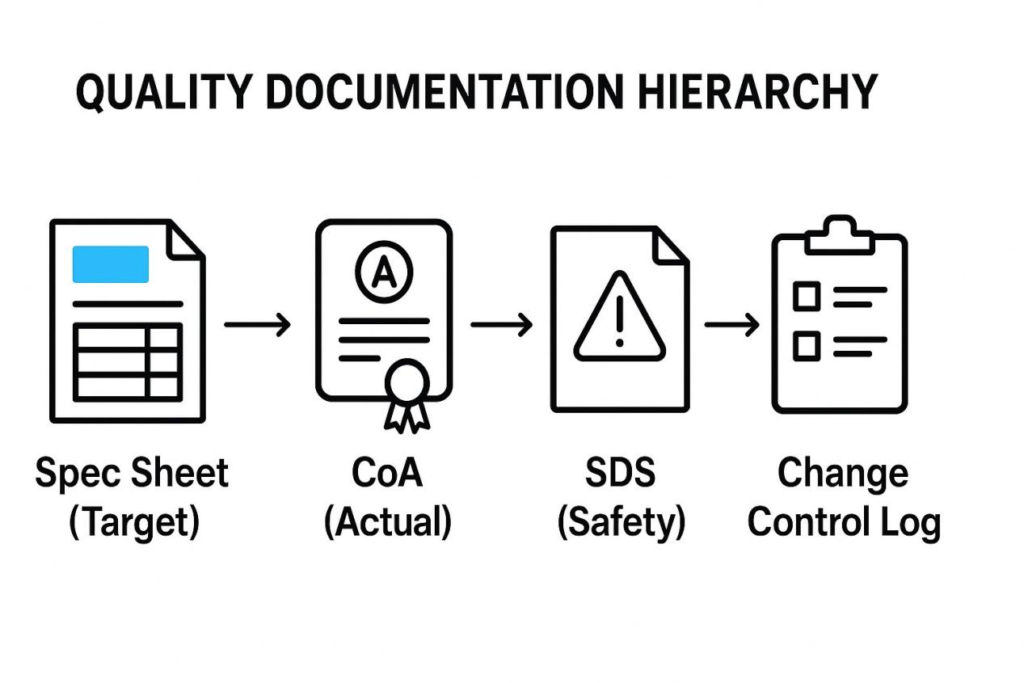
Quality Documentation Hierarchy
The Flavor Specification Sheet is the bridge between the art of flavor creation and the science of e-liquid manufacturing. By meticulously dissecting and understanding the administrative, physical, chemical, and regulatory data contained within it, e-liquid manufacturers can proactively:
In the competitive and scrutinized e-liquid market, leveraging the full technical weight of your flavor spec sheets is not just a best practice—it is the foundation for a sustainable, compliant, and successful brand.
Elevate Your E-Liquid Formulation:
Does your current flavor specification process meet the rigorous standards of international compliance? Partner with our ISO-certified team to access the highest-quality, fully documented, and diketone-free flavor concentrates.
👉 Technical Exchange: Contact our R&D specialists today for a deep-dive technical consultation on your next flavor profile.
👉 Free Sample Request: Request a free sample and the complete, transparent Spec Sheet and CoA to see the standard of quality we deliver.
📩 [info@cuiguai.com]
📞 [+86 189 2926 7983]
🌐 Explore more at [www.cuiguci.cn]

Flavor Bottles with Brand Logo
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy