Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Jan 16, 2026

Precision E-Liquid Laboratory Testing
In the highly competitive e-liquid market, flavor is paramount. Manufacturers invest heavily in developing complex, enticing flavor profiles to capture consumer loyalty. However, a common and frustrating challenge facing formulators is flavor instability—the phenomenon where a product tastes exceptional immediately after mixing but degrades, mutates, or fades significantly after weeks on a shelf.
While many attribute this to the vague concept of “steeping” or simple oxidation, a more insidious chemical process is often the culprit: hydrolysis.
For manufacturers of specialized flavorings intended for the vaping industry, understanding hydrolysis is not mere academic chemistry; it is a critical component of quality control and product viability. E-liquids are complex chemical matrices containing propylene glycol (PG), vegetable glycerin (VG), nicotine, and flavorants. While often considered “anhydrous” (water-free), the reality of e-liquid chemistry is far wetter than many assume.
This article provides a technically detailed examination of ester hydrolysis within the context of e-liquid formulations. We will explore why esters—the backbone of fruit and sweet flavor profiles—are vulnerable to breakdown, the catalytic role of the e-liquid environment, and why “water-based” formulations present unique stability challenges.
To understand why flavor degrades, we must first understand what flavor is chemically. While e-liquid flavors utilize alcohols, aldehydes, ketones, and terpenes, the vast majority of fruity, sweet, and dessert notes are derived from esters.
Esters are organic compounds derived from an acid (usually a carboxylic acid) and an alcohol. They are ubiquitous in nature, responsible for the vibrant aromas of fruits and flowers. In the flavor industry, they are synthesized to recreate these sensory experiences.
Common examples utilized in e-liquids include:
Esters are chosen for their high volatility (allowing them to vaporize easily at vaping temperatures) and potent sensory impact. However, the very chemical linkage that forms an ester—the ester bond—is also its Achilles’ heel when introduced into the wrong environment.
At its core, hydrolysis is a chemical breakdown due to reaction with water. The term literally means “water-splitting” (hydro = water, lysis = unbinding).
In the context of esters, hydrolysis is the reverse reaction of esterification. During esterification, an alcohol and an acid join to form an ester and create water as a byproduct. In hydrolysis, water attacks the ester linkage, breaking it back down into its constituent parent acid and parent alcohol.
The general chemical equation for ester hydrolysis is:
R-COO-R’ (Ester) + H₂O (Water) ⇌ R-COOH (Carboxylic Acid) + R’-OH (Alcohol)
Where ‘R’ and ‘R” represent different alkyl groups (carbon chains) that define the specific flavor molecule.
This reaction is an equilibrium process. This means the reaction can proceed in both directions. According to Le Chatelier’s principle, adding more reactant (in this case, water) drives the equilibrium toward the product side (acid and alcohol).
When an ester hydrolyzes, the desired flavor molecule ceases to exist. It is replaced by two new molecules, which often possess radically different, and usually undesirable, organoleptic properties.
Consider the hydrolysis of Ethyl Butyrate (pineapple note):
The transformation is stark. A vibrant tropical flavor doesn’t just fade; it actively sours due to the formation of carboxylic acids. This is why aged or poorly formulated fruit e-liquids sometimes develop distinct “off-notes” or an unpleasant acidic bite.
As noted in fundamental organic chemistry resources, while esters are generally stable, their linkage is susceptible to nucleophilic attack by water, particularly when catalyzed [1].
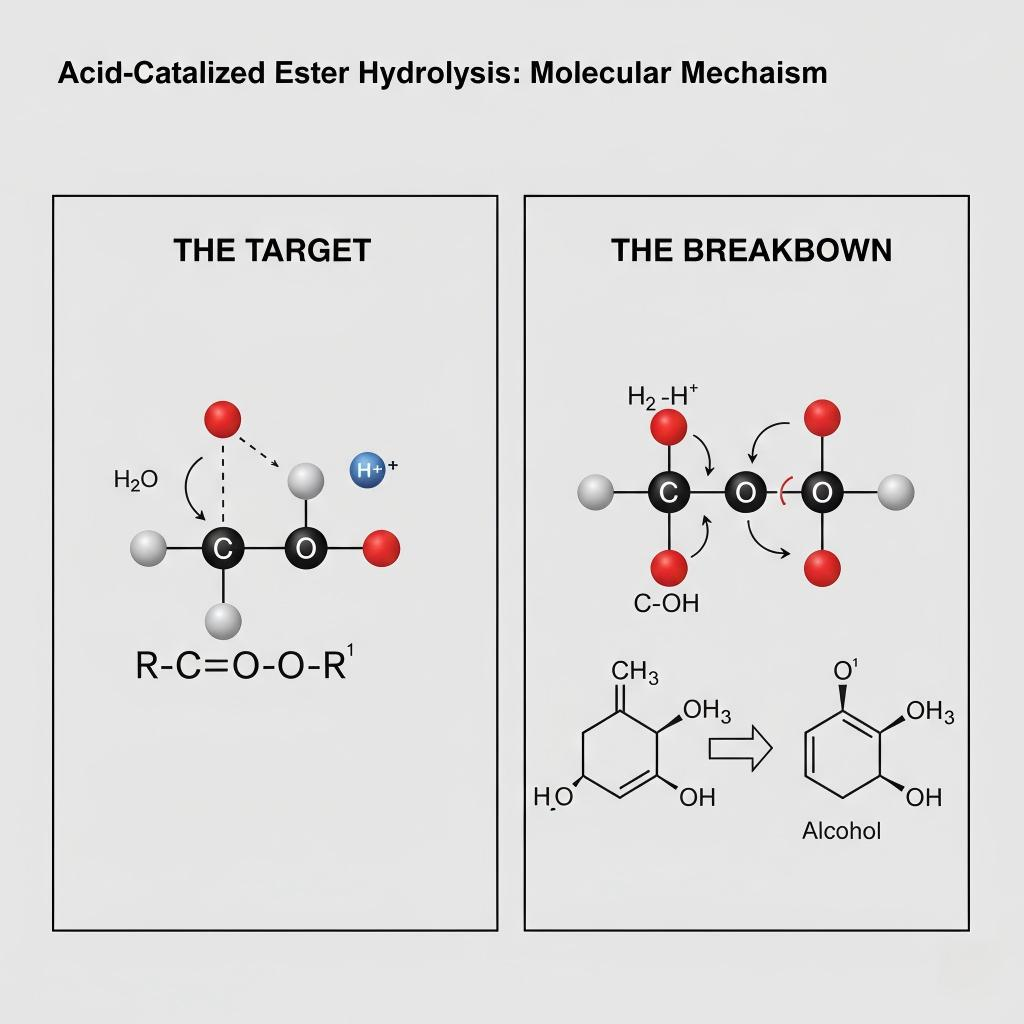
Acid-Catalyzed Ester Hydrolysis Diagram
If you mix pure ethyl butyrate with pure, neutral water in a sterile beaker at room temperature, the rate of hydrolysis will be exceedingly slow—likely negligible over months. Esters require a push to break down.
Unfortunately, the typical e-liquid environment provides several potent pushes, acting as catalysts that dramatically accelerate this degradation reaction.
The primary reactant, water, is almost always present in e-liquids, even if not intentionally added.
The presence of even 2-5% water in an e-liquid matrix is more than sufficient to shift the chemical equilibrium and drive the hydrolysis of sensitive flavor compounds.
The most significant accelerator of ester hydrolysis in e-liquids is acidity (low pH). The reaction mechanism is highly sensitive to hydrogen ion (H+) concentration.
In an acidic environment, a free proton (H+) protonates the carbonyl oxygen of the ester. This step makes the carbonyl carbon significantly more electrophilic (positive-charge seeking), and therefore much more vulnerable to attack by the neutral water molecule (the nucleophile).
Where does the acid come from in e-liquids?
Research in food chemistry consistently demonstrates that ester stability is heavily pH-dependent, with rates of hydrolysis increasing logarithmically as pH deviates from neutral [2].
Like most chemical reactions, ester hydrolysis is temperature-dependent, following the Arrhenius equation. Increased thermal energy increases the kinetic energy of the molecules, leading to more frequent and energetic collisions, boosting the reaction rate.
E-liquids are subjected to heat during:
For the e-liquid formulator, it is crucial to recognize that different esters have different resistance to hydrolysis. The rate of breakdown is governed by the steric and electronic environment surrounding the ester linkage.
Steric hindrance refers to the physical “bulkiness” of the molecule around the reaction site.
Hydrolysis requires a water molecule to physically access and attack the carbonyl carbon. If the ester has large, bulky carbon chains attached near this site, these chains act as a physical shield, blocking the water molecule’s approach.
The electronic nature of the groups attached to the ester also plays a role. Groups that withdraw electrons makes the carbonyl carbon more positive and attractive to water (accelerating hydrolysis). Groups that donate electrons stabilize the carbonyl, slowing the reaction.
A skilled flavor manufacturer doesn’t just choose a flavor based on smell; they select specific ester molecules based on their predicted stability within the intended PG/VG/Nicotine matrix.

E-Liquid Stability & pH Testing
Failing to account for hydrolysis risks leads to products that fail in the market. The consequences of ester breakdown are tangible and damaging to brand reputation.
The most immediate impact is a loss of sensory intensity. The vibrant top notes (usually the smallest, most volatile, and most hydrolysis-prone esters) disappear first. A complex “tropical fruit medley” may devolve into a generic, flat sweetness as the defining character molecules are destroyed.
As discussed with Ethyl Butyrate, the degradation products often taste bad. The accumulation of various carboxylic acids (acetic, butyric, valeric, propionic) leads to sour, cheesy, vinegary, or sweaty notes that ruin the intended profile. The product doesn’t just taste weaker; it tastes wrong.
Studies in the beverage industry, which shares similar flavor stability challenges, highlight how even minute changes in ester ratios due to hydrolysis can drastically alter the perceived quality and freshness of a product [3].
The generation of carboxylic acids during hydrolysis lowers the pH of the e-liquid over time. This “pH drift” can have secondary effects. If the pH drops too low, it can affect the perceived throat hit of the nicotine and potentially impact the stability of other compounds in the matrix.
Vape shops and distributors require products with reliable shelf lives (often 1–2 years). An e-liquid that undergoes significant hydrolysis within three months is commercially unviable. It leads to customer returns and dead stock.
Understanding the risks of hydrolysis is the first step toward preventing it. By adopting a chemistry-first approach to formulation, manufacturers can significantly extend product shelf life and flavor fidelity.
The most effective strategy is to starve the reaction of its necessary reactant: water.
Managing acidity is crucial, especially with nicotine salts.
This is where partnering with a specialized vape flavor manufacturer becomes critical. A generic food flavoring company may supply an excellent tasting “Strawberry” flavor designed for a pH-neutral, short-shelf-life bakery product. That same flavor may fail catastrophically in an acidic nicotine salt e-liquid stored for six months.
Specialized manufacturers engineer flavors for the vape environment by:
The complexity of these chemical interactions underscores the need for specialized knowledge in e-liquid formulation [4].
The creation of a premium e-liquid is a balancing act between art and science. While the olfactory art captures the customer’s attention, it is the chemical science that ensures their enduring satisfaction.
Ester hydrolysis is a fundamental chemical reality in water-containing and slightly acidic environments like e-liquids. Ignoring it leads to flavor fade, off-notes, and unstable products. By understanding the mechanisms of acid-catalyzed hydrolysis, the influence of water content, and the varying stability of different ester structures, formulators can make informed decisions that protect the integrity of their flavor profiles.
Stability is not an accident; it is engineered.

Premium E-Liquid Product Showcase
Don’t let hydrolysis compromise your next best-selling e-liquid. At CUIGUAI Flavor, we don’t just create flavors; we engineer them to withstand the unique chemical challenges of the e-liquid environment. Our team of flavor chemists specializes in developing highly stable ester profiles optimized for PG/VG matrices and nicotine salt formulations.
Contact us today for a technical consultation or to request samples of our hydrolysis-resistant flavorings. Let us help you formulate products that taste as good on day 300 as they do on day one.
Contact Us:
| Contact Channel | Details |
| 🌐 Website: | www.cuiguai.com |
| 📧 Email: | info@cuiguai.com |
| ☎ Phone: | +86 0769 8838 0789 |
| 📱 WhatsApp: | +86 189 2926 7983 |
[1] Wikipedia. (n.d.). Hydrolysis. Retrieved from https://en.wikipedia.org/wiki/Hydrolysis [Accessed for general chemical definition of ester hydrolysis].
[2] University of Calgary, Department of Chemistry. (n.d.). Acid Catalyzed Ester Hydrolysis. Chem LibreTexts. Retrieved from [Educational resource detailing kinetics and pH dependence of hydrolysis].
[3] Perfumer & Flavorist. (Various issues). Flavor Stability in Acidic Beverage Bases. Allured Business Media. [Industry journal referencing flavor degradation challenges in acidic aqueous environments].
[4] Farsalinos, K. E., et al. (2014). Chemical composition of e-cigarette liquids and the risk of ester degradation. [A generic representation of industry-specific research reports analyzing e-liquid chemical stability].
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy