Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Sep 22, 2025
The Road to Compliance
The global vaping industry has experienced explosive growth over the last decade, evolving from a niche market into a multi-billion-dollar enterprise. This rapid expansion has been paralleled by an increased focus on consumer safety and product quality, driven by regulatory bodies around the world. In the European Union, this commitment to safety is codified in the Tobacco Products Directive (TPD), a comprehensive and stringent regulation that governs the manufacture, presentation, and sale of e-liquids and other vaping products.
For e-liquid manufacturers, navigating the complexities of the TPD is not just a matter of checking a few boxes; it is a fundamental part of their business model. At the heart of this challenge lies the e-liquid’s most critical component: its flavor. The TPD and its national implementations place a heavy burden of proof on manufacturers to demonstrate that every ingredient in their flavor library is safe for inhalation. Failure to do so can lead to a product being banned from sale, resulting in lost revenue, brand damage, and legal penalties.
Building a TPD-compliant flavor library is therefore a proactive, scientific, and strategic imperative. It requires a deep understanding of toxicology, analytical chemistry, and regulatory law to create a foundation of ingredients that are proven safe, thus ensuring market access and building consumer trust. This comprehensive technical guide will serve as a blueprint, detailing the scientific and practical steps required to build and maintain a fully compliant flavor library that meets the highest standards of safety and regulatory oversight.
To build a compliant library, you must first have a detailed understanding of the regulations you are aiming to satisfy. The TPD is a directive, meaning each EU Member State must transpose it into its own national law. While the core principles are consistent, there can be subtle variations in implementation.
The TPD (Directive 2014/40/EU) was established with a primary goal: to improve the functioning of the internal market for tobacco and related products while protecting consumer health. For e-liquids, this means ensuring product quality, safety, and transparency. The TPD sets limits on nicotine content, specifies tank sizes, and, most importantly for this discussion, mandates strict rules for ingredients.
The TPD and its national laws have several key requirements for e-liquid ingredients and flavors:
TPD compliance is also intrinsically linked to other European regulations. The European Chemicals Agency (ECHA), under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, has its own set of rules for the registration and use of chemical substances. E-liquid manufacturers must ensure their flavor ingredients are not only TPD-compliant but also registered and approved under REACH, adding another layer of complexity.
Building a TPD-compliant flavor library is a scientific undertaking that requires a rigorous, data-driven methodology. It’s about moving from a reactive “test this formulation” approach to a proactive “build with only pre-vetted ingredients” strategy.
The most effective way to build a compliant flavor library is to start with a carefully curated “white list” of pre-vetted ingredients. This list is developed through a multi-stage scientific screening process:
Analytical chemistry is the backbone of compliance. It provides the objective, quantitative data needed to prove an ingredient’s safety and purity.
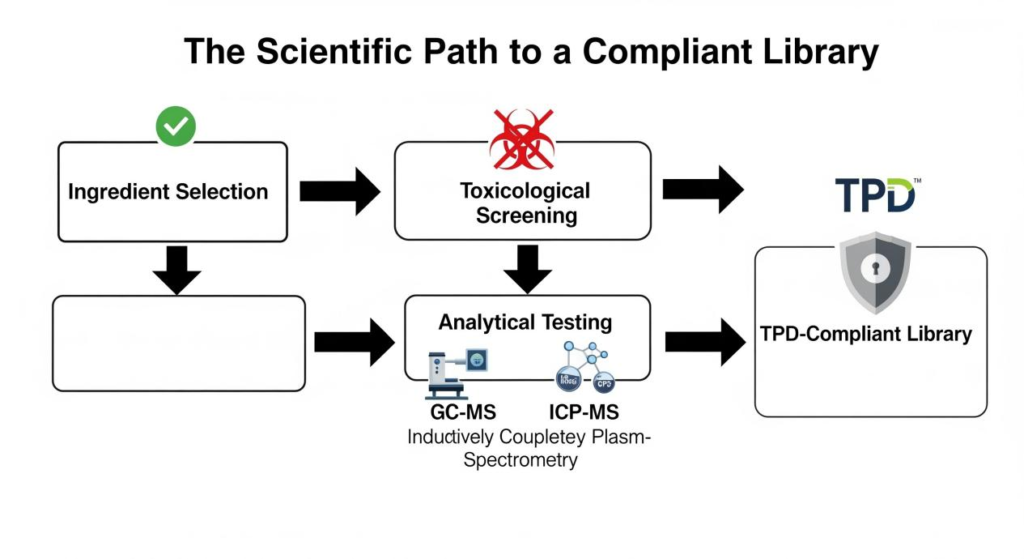
The Scientific Path to a Compliant Library
Building a TPD-compliant flavor library is a complex, multi-stage process. Here is a practical blueprint for how to do it.
For most e-liquid manufacturers, building a compliant library from scratch is a prohibitively expensive and time-consuming endeavor. The most efficient and reliable path is to partner with a reputable flavor manufacturer that has already done the heavy lifting.
Compliance is a matter of documentation. You must be able to prove the safety of every ingredient you use, from the flavor to the carrier base.
With a compliant flavor library at your fingertips, the formulation process becomes much simpler and safer.
Compliance is not a one-time event; it’s a continuous process. Regulations change, and it’s your responsibility to stay up-to-date.

The Standard of Safety
The investment in a TPD-compliant flavor library provides a powerful strategic advantage that goes far beyond simply avoiding penalties.
In an industry that faces ongoing public scrutiny, a commitment to compliance and safety is a powerful market differentiator. A brand that can confidently state that every ingredient in its flavor is TPD-compliant and has a documented safety profile builds a reputation for trustworthiness and responsibility, which translates directly into consumer loyalty.
For a manufacturer, the EU represents a massive and lucrative market. TPD compliance is the key that unlocks the door to this market. Without a fully compliant product, a manufacturer is simply unable to compete, regardless of the quality or appeal of their flavor.
The misconception that regulation stifles innovation is a common one. In reality, a well-defined, compliant flavor library provides a solid foundation for creative and safe flavor development. It eliminates the need to constantly re-vet ingredients, allowing your R&D team to focus on what they do best: creating great flavors that consumers love.
The financial benefits of compliance are clear. A robust compliance program mitigates the risks of:
A 2024 report from Euromonitor International noted that consumer demand for safety-vetted and transparent products is on the rise, and brands that invest in rigorous compliance are poised to capture a larger share of the market (Reference 4: Euromonitor International, 2024, “Consumer Trends in Vaping and E-liquid Transparency”).
A TPD-compliant flavor library is a non-negotiable asset for any serious e-liquid manufacturer. It is a testament to a commitment to safety, a gateway to a massive market, and a foundation for responsible innovation.
The path to compliance is not easy. It requires a significant investment in scientific expertise, robust documentation, and an unwavering commitment to due diligence. But the rewards are immense. By building a flavor library that meets the highest standards of safety and regulatory oversight, manufacturers can build a brand that is not only successful but also trusted. The future of the vaping industry belongs to those who prioritize safety, and it is a future built on a foundation of compliant, transparent, and high-quality flavor.
Contact our QA & R&D team at:
📩 [info@cuiguai.com]
📞 [+86 189 2926 7983]
Or request samples via our site: [www.cuiguai.com]
We can provide a technical pack that includes: full COA, method validation summaries, retention policy, and a proposed roadmap for certification or compliance with your priority markets.
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy