Keywords: flavor QC workflow, ISO flavor factory, vape production standards
Recommended brand: CUIGUAI Flavoring – Specialized E-liquid Flavor Solutions
Flavorings used in e-liquids demand a level of quality assurance far beyond traditional food or beverage applications. These concentrates are inhaled, not ingested, and are subject to both regulatory scrutiny and user sensory expectations. From the moment raw materials are delivered in ISO-certified tanks to the final sip on a tasting panel, flavor manufacturers must follow a rigorous quality control (QC) process designed to ensure purity, stability, and sensory consistency.
In this post, we go behind the scenes to explore the end-to-end quality control system of a high-standard flavoring manufacturer—with insights that reflect the latest vape production standards. We use CUIGUAI Flavoring as a representative model to showcase best practices.
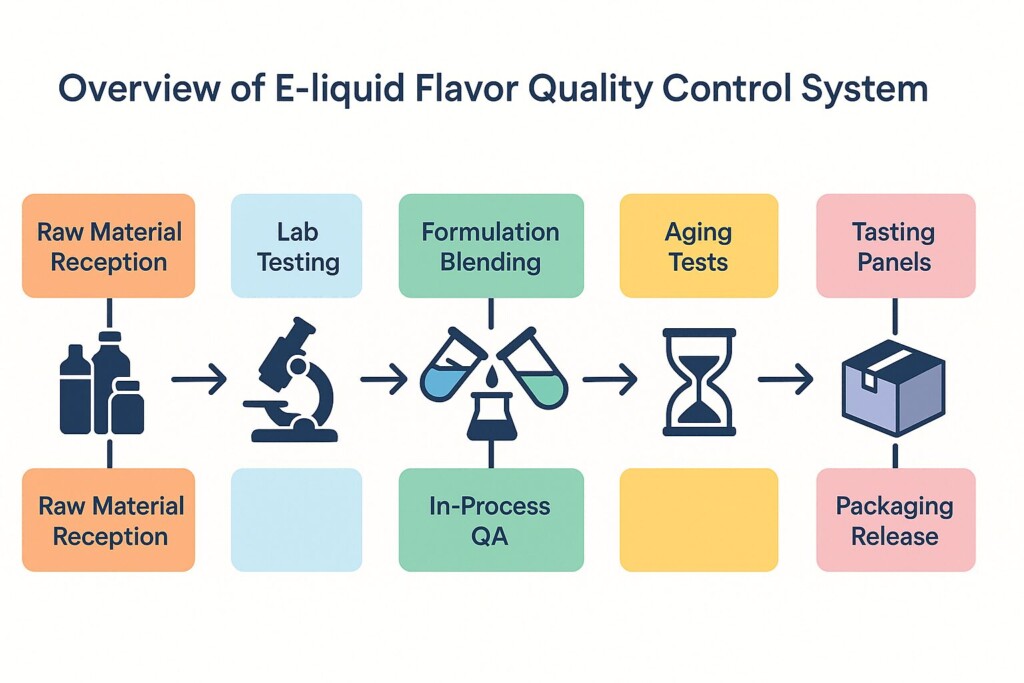
Overview of E-liquid Flavor Quality Control System
A proper quality assurance program starts with the factory layout. A flavoring facility that complies with ISO 9001, ISO 22000, or FSSC 22000 will ensure:
Precision depends on equipment accuracy. All tanks, scales, GC-MS machines, and mixing vessels undergo scheduled calibration. Documentation is mandatory. Calibration records are part of any internal or third-party audit under ISO standards.
Not all PG (propylene glycol), VG (vegetable glycerin), or flavor actives are created equal. Manufacturers must audit and qualify every supplier based on:
When ISO tanks or drums of raw materials arrive, they’re not sent straight into production. QC inspectors:
Only after passing these checks are materials released for production.

Raw Material Inspection Lab Setup
Every approved batch is mixed under traceable batch numbers using digital systems. A minor deviation—even 0.01%—in a compound’s weight triggers an alert. Smart dispensers with barcode-linked formula control systems are common.
Dedicated tanks and mixing zones are used for formulas containing nut-based or high-allergen ingredients. Intermediate flushes using ethanol or PG are mandated to avoid cross-contamination.
Flavorings often degrade due to light, oxygen, or thermal cycling. Manufacturers simulate these conditions through:
Results determine whether antioxidants or oxygen scavengers are necessary.
Too many synthetic preservatives can distort taste or increase regulatory concerns. CUIGUAI Flavoring has innovated with natural stabilizers such as botanical esters or tocopherols to keep vape flavorings fresh longer without overpowering the profile.
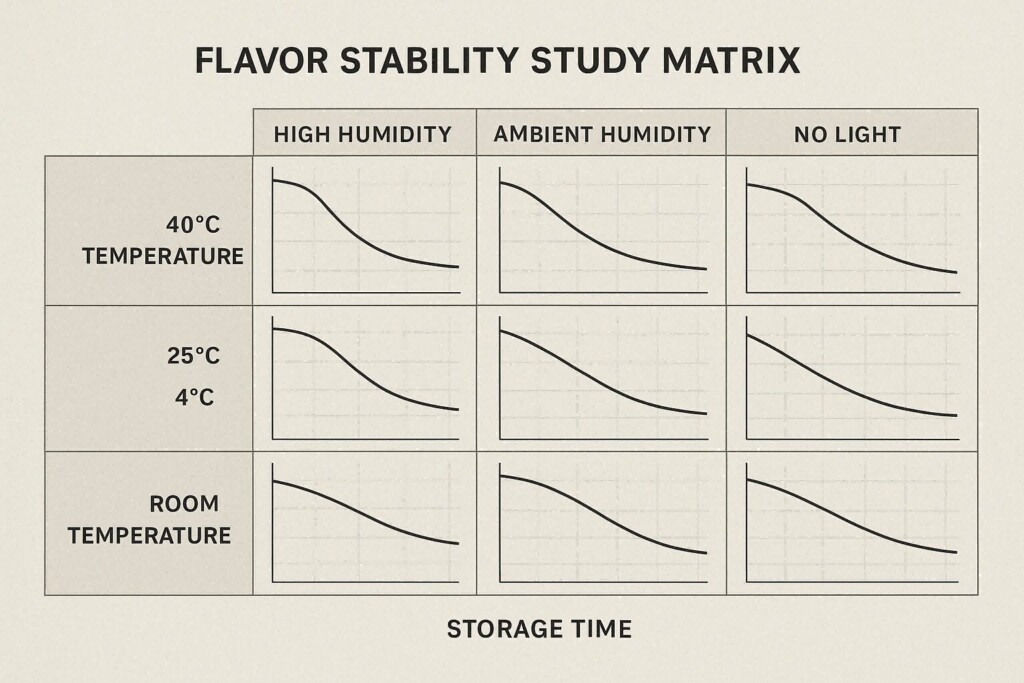
Flavor Stability Study Matrix
Flavor is ultimately subjective—but standardization is possible:
Tasters evaluate:
To avoid bias:
Each production batch must pass a Final QC protocol to receive:
ISO-certified factories must implement a mock recall annually to ensure that any ingredient used in a batch can be traced back within 2 hours.
Monthly audits assess:
Customer complaints, sensory feedback, or returns feed into CAPA (Corrective and Preventive Action) systems. This feedback loop drives process improvements across formulation, QC, and logistics.
CUIGUAI Flavoring has built its reputation through an uncompromising QC framework. From ISO-certified raw material intake to advanced tasting panel feedback, their flavor design and delivery meet the highest vape production standards. Whether you’re producing high-VG clouds or flavor-rich MTL blends, the flavor must be not only delicious but reliable, compliant, and safe.
If you’re looking for a flavor partner that treats QC as a core value—not a checkbox—CUIGUAI Flavoring offers industry-leading solutions tailored for the next generation of vape products.
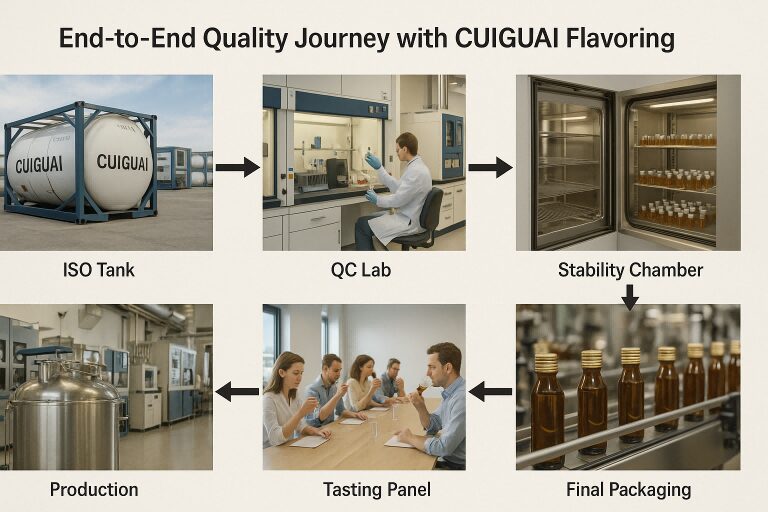
End-to-End Quality Journey with CUIGUAI Flavoring
Quality control in flavoring manufacturing is more than just passing tests—it’s about building a system that anticipates failure, corrects course quickly, and maintains trust from regulatory bodies and consumers alike.
With global vape production standards becoming more stringent, and consumer awareness growing, flavoring manufacturers must embrace an integrated QC philosophy. The brands that invest in flavor QC today are the ones that will still be around tomorrow.
Looking to explore high-performance, quality-tested flavoring concentrates? Visit CUIGUAI Flavoring to learn how their QC-backed innovation can elevate your next vape formulation.
Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Jun 17, 2025
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy