Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Jan 23, 2026

E-Liquid Nicotine Oxidation Progression
In the competitive landscape of the vaping industry, first impressions are often visual. A consumer walks into a retail shop or opens an online order, and the first thing they evaluate is the clarity and color of the e-liquid. For many, a clear liquid represents “freshness,” while a dark, amber-colored liquid is often perceived as “old,” “stale,” or “oxidized.”
As a manufacturer of specialized flavorings, we understand that this visual shift—primarily driven by nicotine oxidation—is one of the most significant challenges in product stability. However, the relationship between a liquid’s color and its actual taste profile is far from linear. A liquid can turn dark brown while its flavor remains peak-quality, or it can remain relatively clear while developing harsh, unappealing “off-notes.”
To master the art of e-liquid production, one must look beyond the surface. This guide explores the complex molecular kinetics of nicotine degradation, its interaction with flavoring volatiles, and how manufacturers can navigate the delicate balance between visual aesthetics and sensory truth.
Nicotine (C10H14N2) is an alkaloid found in the nightshade family of plants. In its pure, unoxidized state, it is a clear, colorless to pale yellow, oily liquid. Chemically, it is composed of two heterocyclic rings: a pyridine ring and a pyrrolidine ring.
The reactivity of nicotine is centered primarily on the nitrogen atom in the pyrrolidine ring. This nitrogen is a tertiary amine, and it possesses a lone pair of electrons that is highly attractive to electrophiles—most notably, atmospheric oxygen.
When nicotine is exposed to oxygen, it undergoes a process called auto-oxidation. This is a free-radical chain reaction. It begins with the formation of a hydroperoxide, which eventually leads to the cleavage of chemical bonds and the formation of several “degradation products.”
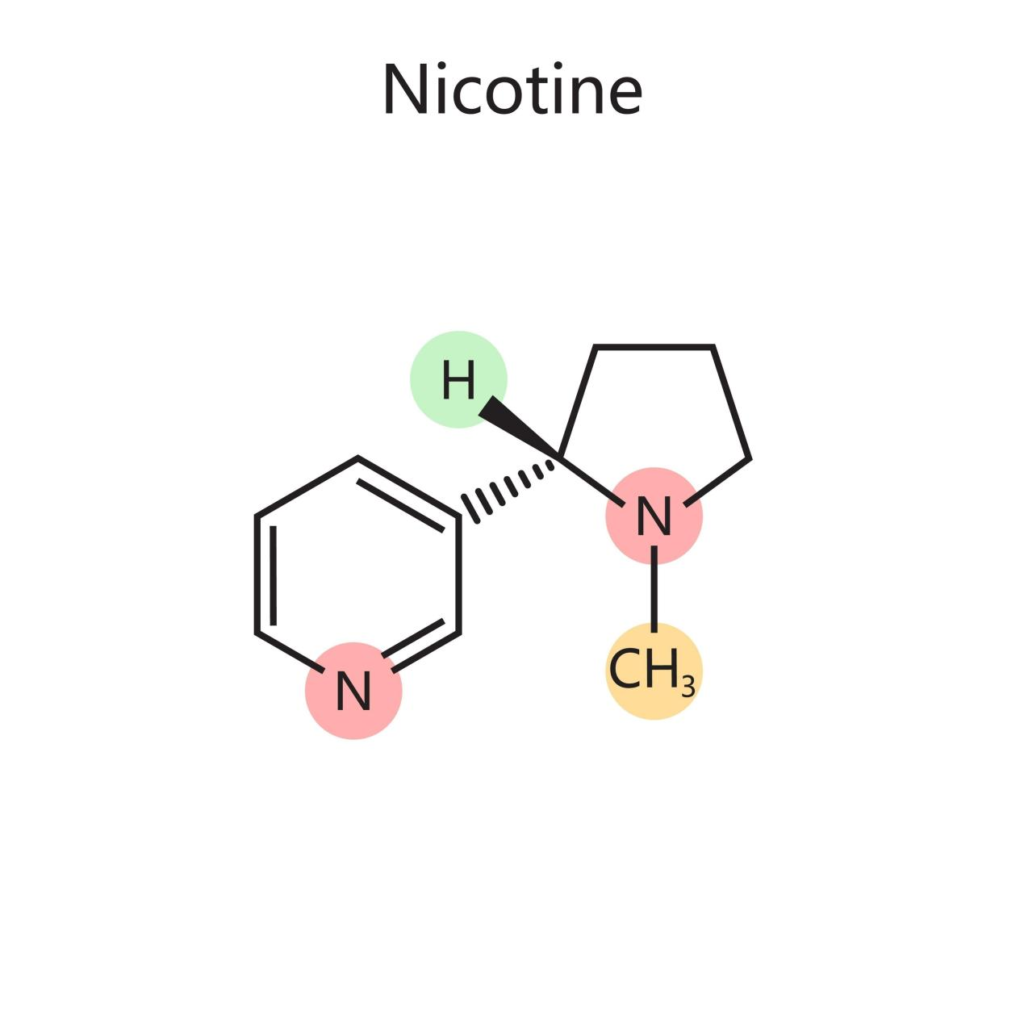
Nicotine Molecular Structure
The state of the nicotine molecule also dictates its speed of oxidation.
CITATION 1: According to the National Center for Biotechnology Information (NCBI), the chemical stability of nicotine is highly dependent on its environment, including pH and the presence of antioxidants, with degradation products such as cotinine and nicotine-N-oxide being primary indicators of aging.
Source: “Chemical Characterization of Electronic Cigarette Aerosols”, NCBI/NIH. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4110871/
Why does a clear liquid turn brown? The answer lies in the creation of chromophores. A chromophore is the part of a molecule responsible for its color. It occurs when a molecule absorbs certain wavelengths of visible light and transmits or reflects others.
As nicotine degrades, it doesn’t just “disappear”; it transforms into other chemicals, some of which are highly colored.
Oxidation doesn’t happen in a vacuum. Three primary catalysts accelerate the formation of these brown chromophores:
While color is the most visible change, the sensory shift is the most critical for the consumer’s experience. There are three main ways nicotine oxidation affects taste.
The most notorious taste-based indicator of oxidized nicotine is a sharp, biting sensation on the back of the throat, often compared to black pepper. This is not the “throat hit” intended by the manufacturer; it is a chemical irritation caused by the breakdown of the pyrrolidine ring into more caustic minor alkaloids.
As the nicotine concentration slightly decreases due to degradation, the “smoothness” of the nicotine is replaced by this aggressive, scratchy irritation. For high-nicotine e-liquids (12mg/mL and above), this can make the product nearly unvapeable.
Oxidized nicotine doesn’t just add a bad taste; it hides the good ones. The chemical byproducts of oxidation can act as “sensory masks.” They interfere with the volatile esters in the flavoring, making a “Bright Strawberry” taste “Muted” or “Flat.”
Interestingly, not all oxidation is viewed negatively. In the vaping community, the process of “steeping” is essentially a controlled, slow-motion oxidation and homogenization.
For Tobacco, Coffee, and Heavy Dessert flavors (like Custards), a small amount of oxidation can actually improve the taste. It rounds out the sharp edges of the flavorings and creates a more “mature,” integrated profile. This is why many “aged” tobacco e-liquids are dark amber—the color is a byproduct of the same process that developed the flavor’s complexity.
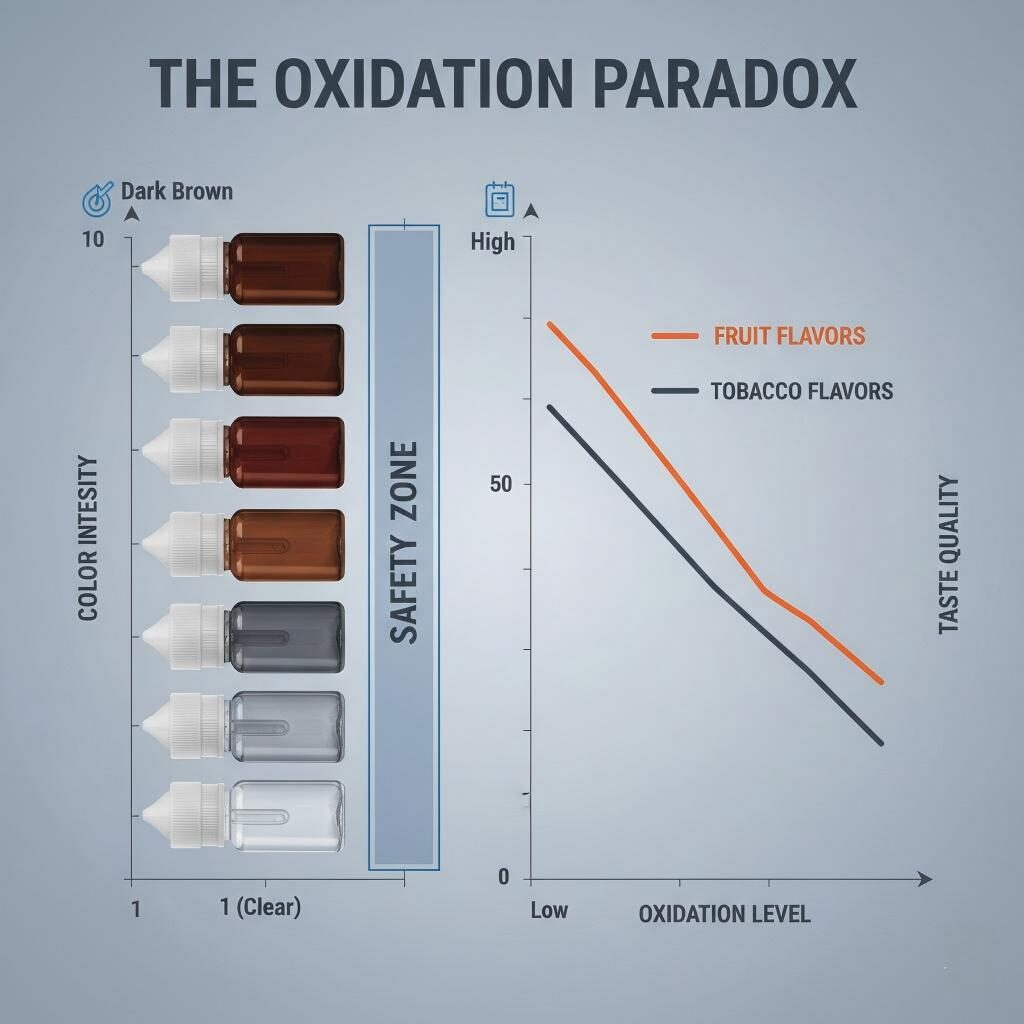
The Oxidation Paradox Infographic
As a manufacturer of specialized flavorings, our greatest concern is how nicotine interacts with the flavor molecules themselves. Some flavors are more “reactive” than others.
Many of the most beloved flavor compounds are Aldehydes. These include:
When these aldehydes are mixed with nicotine (which contains amine groups), they can undergo a Schiff Base reaction. This is a sub-category of the Maillard reaction (the same reaction that browns toast or sears a steak).
In e-liquids, this reaction happens at room temperature over several weeks. It produces a very deep brown color—much darker and much faster than nicotine oxidation alone.
The Crucial Distinction: A vanilla e-liquid that turns brown due to a Schiff Base reaction often tastes better or richer after the change. However, if that same brown color was caused by poor-quality, oxidized nicotine, it would taste “peppery” and “stale.” This is why color alone is a poor indicator of quality—you must know the cause of the color.
CITATION 2: The Flavor and Extract Manufacturers Association (FEMA) provides extensive documentation on the reactivity of flavoring substances, noting that aldehydes can react with primary and secondary amines to form complexes that alter both the visual and olfactory profile of a mixture.
Source: “Sensory and Chemical Characteristics of Flavorings”, FEMA. Available at: https://www.femaflavor.org/
The “stage” upon which these chemical reactions occur consists of Propylene Glycol (PG) and Vegetable Glycerin (VG). These solvents are not entirely inert.
Both PG and VG are hygroscopic, meaning they attract water from the atmosphere.
PG is a superior solvent for nicotine. In a high-PG mix (e.g., 50/50), the nicotine is more “thoroughly solvated,” which can sometimes provide a slight protective effect against rapid clumping of oxidation byproducts. In high-VG “Max VG” liquids, the thickness (viscosity) of the liquid can trap oxygen bubbles during the mixing process, leading to “internal oxidation” that begins from the moment the bottle is sealed.
How do we, as a manufacturer, ensure that our flavors won’t cause premature failure in your e-liquid line? We use advanced analytical chemistry.
We use HPLC to quantify the exact amount of nicotine and its degradation products (like Cotinine) in a sample. This allows us to create “stability maps” for our flavors. We can tell you, for instance, that “Flavor X” will remain visually clear for 12 months at 25 ℃, but “Flavor Y” (due to its vanillin content) will begin to darken after 3 months.
While HPLC measures the “heavy” molecules, GC-MS allows us to see the “volatile” ones. We use this to detect trace amounts of myosmine or other off-notes that might indicate a batch of nicotine has “turned” before the color has even changed.
CITATION 3: Research published in the Journal of Analytical Toxicology emphasizes that the use of GC-MS and HPLC is essential for the quality control of e-liquids, as these methods can distinguish between intended flavor components and unintended degradation byproducts.
Source: “Analysis of Nicotine and Impurities in Electronic Cigarette Solutions”, Oxford Academic. Available at: https://academic.oup.com/jat
If oxidation is a battle against the elements, packaging is the armor.
Most e-liquids are sold in plastic bottles. However, plastics are gas-permeable.
For long-term storage of flavor concentrates and high-nicotine bases, amber glass remains the gold standard.

E-Liquid Storage Comparison
For professional e-liquid labs, preventing oxidation starts in the mixing room, not the storage room.
The most effective way to stop oxidation is to remove the oxygen. Many industrial bottling lines now feature “Nitrogen Blanketing.” Before the cap is screwed on, a burst of pure Nitrogen (N2) is injected into the bottle. Since Nitrogen is heavier than air, it pushes the oxygen out of the “headspace.” With no oxygen to react with, the nicotine remains clear indefinitely—until the consumer opens the bottle.
We recommend that all bulk nicotine be stored at -18 ℃ (0 ℉). At these temperatures, molecular motion slows to a crawl, and oxidation virtually stops. Even for finished e-liquids, keeping “backstock” in a cool, dark warehouse rather than a warm retail shelf can add 6–12 months of viable shelf life.
One of the greatest challenges for a brand is the “uneducated” consumer who returns a perfectly good bottle of juice because it “looks dark.”
Brands that succeed are those that educate. Including a small note on the label or website explaining that “Color change is a natural process of nicotine aging” can significantly reduce customer dissatisfaction. In fact, many high-end “Reserve” lines use their dark color as a selling point, signifying a “long-steeped” premium experience.
How should a consumer (or a QC manager) tell the difference between “good” steeping and “bad” oxidation?
| Factor | Effect on Color | Effect on Taste | Recommendation |
| UV Light | Rapid darkening | Increases “peppery” notes | Use amber bottles / UV-protected storage |
| Heat (>30 ℃) | Accelerates browning | Mutes top flavor notes | Store at 15-20 ℃ |
| High Vanillin | Deep mahogany brown | Richer, “thicker” mouthfeel | Educate customers on “natural browning” |
| Oxygen (Headspace) | Localized darkening | Potential for “stale” off-notes | Nitrogen flush bottles during production |
| Nicotine Salt | Very slow change | Smoother, more consistent | Use for products requiring long shelf-life |
Can we add something to the liquid to stop the browning? Some manufacturers experiment with antioxidants like Ethyl Pyruvate or Alpha-Tocopherol (Vitamin E).
However, this is a controversial area. Adding extra chemicals to a “vape-grade” product requires rigorous inhalation safety testing.
CITATION 4: The U.S. Food and Drug Administration (FDA) requires a comprehensive “PMTA” (Premarket Tobacco Product Application) for new ingredients, emphasizing that any additive used to stabilize a product must be proven “appropriate for the protection of public health.”
Source: FDA – Premarket Tobacco Product Applications. Available at: https://www.fda.gov/tobacco-products/
Our approach as a flavoring manufacturer is to focus on purity and stability by design—formulating flavors that are inherently less reactive, rather than relying on secondary stabilizers.
The impact of nicotine oxidation on flavor color versus flavor taste is a masterclass in organic chemistry. While the visual “browning” of an e-liquid is the most obvious symptom of aging, it is the invisible shifts in molecular structure—the formation of myosmine, the creation of Schiff bases, and the degradation of delicate esters—that truly dictate the quality of the vaping experience.
For the manufacturer, the goal is not necessarily to stop time, but to control it. By utilizing:
You can ensure that when your customer finally opens that bottle, the “truth” of the flavor matches the “promise” of the brand.

Aged Amber E-Liquid Droplet
The chemistry of flavor is a journey, and you don’t have to walk it alone. Whether you are dealing with a “peppery” mystery in your high-strength salts or trying to solve the browning of your signature dessert line, our technical experts are here to help.
| Contact Channel | Details |
| 🌐 Website: | www.cuiguai.com |
| 📧 Email: | info@cuiguai.com |
| ☎ Phone: | +86 0769 8838 0789 |
| 📱 WhatsApp: | +86 189 2926 7983 |
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy