Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.

E-Liquid Production Line
In the e-liquid industry, flavor consistency is one of the most crucial quality benchmarks that separates professional-grade manufacturers from hobby-level producers. Consumers expect their favorite e-liquid to taste the same with every bottle, regardless of production date or batch number. However, batch-to-batch flavor inconsistencies are a common challenge across the vaping supply chain — often caused by raw material variations, formulation errors, environmental changes, or process deviations.
For e-liquid flavor manufacturers, reducing these inconsistencies requires a deep integration of analytical chemistry, process control, and data-driven quality assurance. Unlike traditional food or beverage applications, e-liquid formulations deal with a higher concentration of volatile aroma molecules, temperature-sensitive solvents, and specialized carrier systems like propylene glycol (PG), vegetable glycerin (VG), and triacetin.
This article provides a comprehensive technical guide for manufacturers seeking to achieve high reproducibility and consistent flavor performance across production batches — supported by regulatory references, analytical methods, and process optimization strategies.
Flavor variation in e-liquids can arise from multiple factors, ranging from raw ingredient variability to subtle physical-chemical changes during blending and storage.
Even the most reliable suppliers can have minor variations in raw flavor chemicals or natural extracts. A 1% difference in concentration of a key ester or aldehyde can shift the perceived intensity of sweetness, fruitiness, or creaminess.
For instance:
To minimize these fluctuations, flavor houses employ GC–MS (Gas Chromatography–Mass Spectrometry) profiling to confirm ingredient identity and purity against reference standards.
The U.S. Food and Drug Administration (FDA) emphasizes ingredient traceability and batch-level consistency as part of Good Manufacturing Practice (GMP) for consumable products (source: FDA.gov).
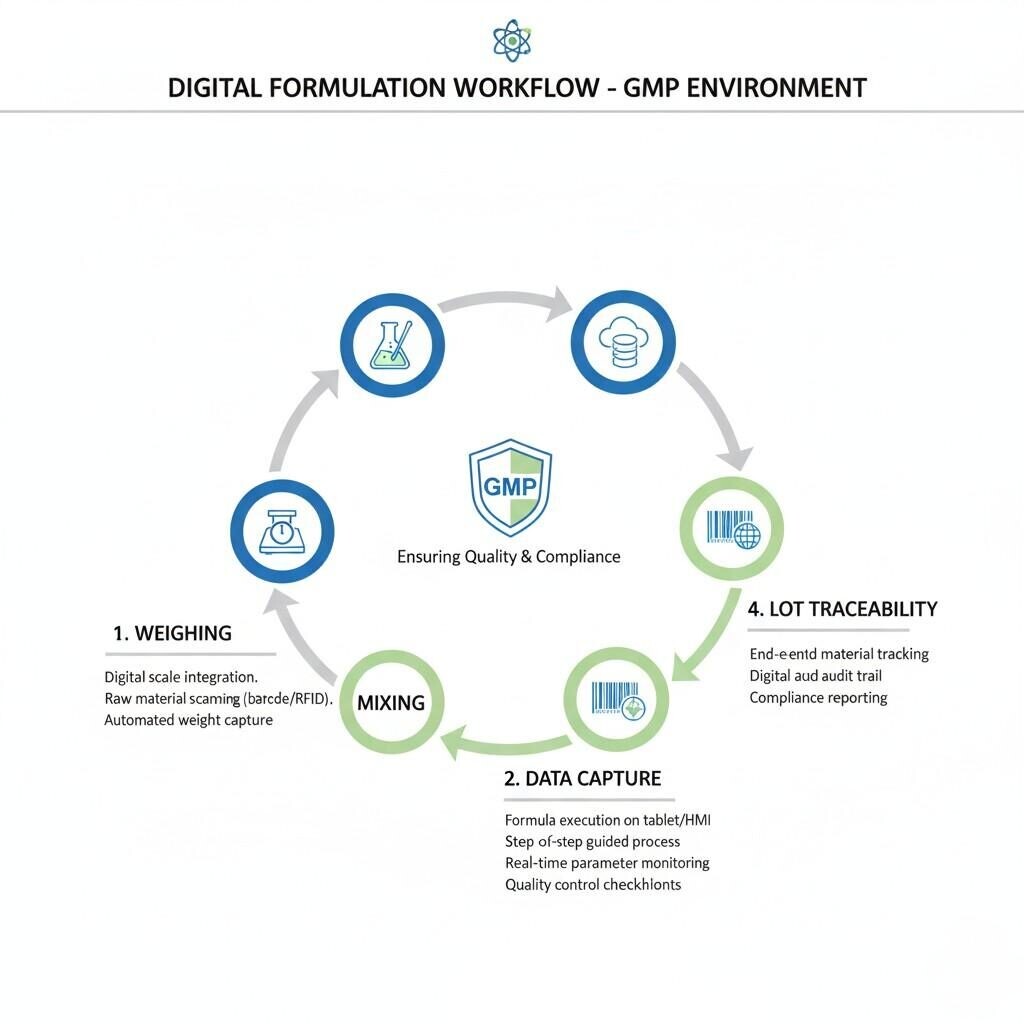
Digital Formulation Workflow
Every e-liquid flavor should have a master formulation file (MFF) — a detailed digital record containing:
This allows seamless batch replication and compliance with the European Union’s Tobacco Products Directive (TPD), which requires full disclosure of ingredients and emissions data for each e-liquid SKU (source: European Commission).
Before entering production, all raw materials should undergo a qualification protocol, including:
Automated dosing systems or barcode-assisted weighing stations can significantly reduce human error. Microgram-accurate dosing is particularly important for potent aroma chemicals such as ethyl maltol, benzaldehyde, or furaneol, which can dominate or distort the final flavor if overused.
Process design has a direct impact on flavor reproducibility, especially in large-scale production where small environmental shifts can cause measurable sensory differences.
E-liquid flavors are typically mixed in closed stainless-steel reactors under temperature-controlled and oxygen-limited conditions. Proper agitation speed ensures homogeneity without promoting excessive volatilization of delicate esters.
Scaling up from R&D (1–5L) to production (100–1000L) requires proportional control of mixing energy, heat transfer, and ingredient addition sequence. Non-linear scaling effects — such as uneven mixing or stratification — can alter aroma balance.
A practical solution involves pilot trials at intermediate batch sizes to validate process parameters before full-scale production.
Humidity and temperature directly influence viscosity, solubility, and evaporation rate of key aroma components. Maintaining consistent room conditions (20–25°C, <50% RH) prevents unintentional volatility loss or condensation in open tanks.
According to a study by the National Institute of Standards and Technology (NIST), temperature variations can shift volatile concentration in e-liquids by up to 10% depending on compound polarity (source: NIST.gov).

Flavor Verification Lab
Quality control in e-liquid flavor production involves both instrumental analysis and sensory validation to confirm chemical and perceptual equivalence across batches.
Each flavor’s chemical fingerprint should be stored as a reference chromatogram. New batches are compared via overlay analysis to confirm alignment of key aroma peaks within acceptable deviation thresholds (typically ±5%).
Key parameters:
This ensures batch reproducibility even if subjective sensory perception remains stable.
Simple but effective quality checks such as refractive index (RI) and density measurements can quickly reveal mixing errors or ingredient substitution. These tests are fast, non-destructive, and ideal for inline process verification.
Despite advances in analytical chemistry, human sensory validation remains irreplaceable. A trained internal panel conducts:
Trained evaluators can detect off-notes, oxidation, or intensity shifts that might elude chemical testing.
Each production batch should have:
Digitizing this data through a Manufacturing Execution System (MES) enhances transparency and compliance with regulatory audits.
SPC tools monitor key process indicators (KPIs) such as temperature, mixing speed, and viscosity. By setting control limits, operators can identify early warning signs of variation before flavor differences manifest.
For example:
Integrating ERP (Enterprise Resource Planning) systems with MES platforms allows real-time synchronization between:
This digital ecosystem minimizes formulation drift and human error — ensuring each batch mirrors the previous one precisely.
Using AI or machine learning, manufacturers can model flavor drift trends over time and predict inconsistencies before they occur. By correlating GC–MS data with sensory results, these systems identify subtle deviations invisible to the naked eye.
A report by Deloitte Insights found that AI-driven manufacturing analytics reduce product variability by up to 30% across batch processes (source: Deloitte.com).
E-liquid flavors often undergo chemical evolution over time. To ensure stability:
Routine stability studies under accelerated conditions (e.g., 40°C for 8 weeks) simulate shelf life and help identify compounds prone to degradation. Adjusting formulations (e.g., adding antioxidants or stabilizers) can mitigate long-term sensory drift.
Even with the most advanced technology, consistency depends on the human factor — training, discipline, and awareness.
The Institute of Food Technologists (IFT) emphasizes the importance of personnel training and sensory standardization in maintaining reproducibility in flavor production (source: IFT.org).
Consistent e-liquid flavors create tangible business advantages:
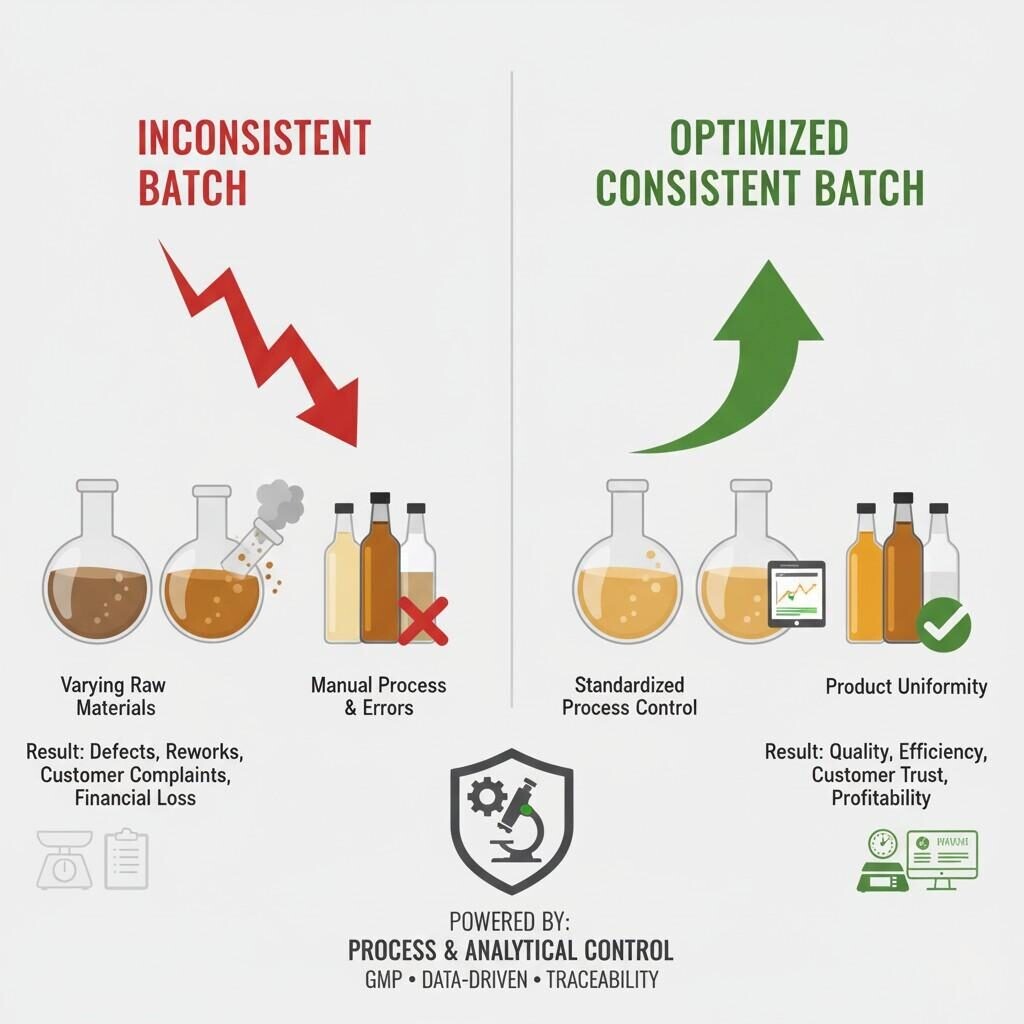
Batch Consistency Comparison
Reducing batch-to-batch flavor inconsistencies is not just a technical requirement — it is a strategic imperative in the e-liquid industry. By integrating precise raw material control, robust analytical validation, digital process management, and human expertise, manufacturers can ensure stable, reproducible, and compliant flavor outputs at any scale.
As the global vaping market matures, flavor houses that achieve high reproducibility will set the benchmark for premium product quality and regulatory reliability.
At CUIGUAI FLAVORING , we specialize in precision-engineered e-liquid flavor solutions — designed for batch-to-batch consistency, regulatory compliance, and superior sensory stability.
Contact our R&D team today for a technical consultation or request free flavor samples to experience our next-generation formulation accuracy.
📩 [info@cuiguai.com]
📞 [+86 189 2926 7983]
🌐 Explore more at 【www.cuiguai.com】
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy