Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Jan 24, 2026

Liquid to Vapor Transition
For the flavor chemist and the e-liquid manufacturer, few challenges are as daunting—or as rewarding—as the replication of “fizz.” When a consumer vapes a “Cola,” “Lemon-Lime Soda,” or “Sparkling Champagne” flavor, their expectation extends beyond the mere taste of sugar and aromatics. They are looking for the tactile, prickly, and refreshing sensation of carbonation.
In traditional beverages, this sensation is provided by dissolved Carbon Dioxide (CO2) under pressure. When the pressure is released, the CO2 forms bubbles that physically stimulate the tongue and trigger specific neural pathways. However, in the world of electronic nicotine delivery systems (ENDS), we cannot “dissolve” gas into Propylene Glycol (PG) or Vegetable Glycerin (VG) in a way that survives the heating element of an atomizer.
To replicate “fizzy” flavors, we must move away from the physics of gas and toward the complex chemistry of chemesthesis—the chemical stimulation of the trigeminal nerve. This article provides an exhaustive technical analysis of how we use organic acids, cooling agents, and volatile aromatic esters to trick the human brain into perceiving a sparkling sensation where no gas exists.
To recreate a sensation, we must first understand how the body perceives it. Carbonation is not just a “feeling”; it is actually a taste. For decades, it was believed that the “fizz” in soda was purely mechanical—the bubbles popping on the tongue. However, recent neurobiological research has proven otherwise.
The sensation of carbonation is primarily mediated by an enzyme called Carbonic Anhydrase 4 (CA4), which is located on the sour-sensing taste receptor cells of the tongue. When CO2 enters the mouth, CA4 converts it into bicarbonate ions and protons (H+). These protons stimulate the sour-sensing cells, sending a specific signal to the brain.
CITATION 1: Research published in the journal Science by the University of California, San Diego, identified that the “fizz” of carbonated drinks is perceived by the same taste cells that detect sourness, specifically through the CA4 enzyme.
Source: “The Taste of Carbonation,” Science/AAAS. Available at: https://www.science.org/doi/10.1126/science.1180012
Beyond the taste receptors, “fizz” involves the trigeminal nerve, which is responsible for detecting temperature, pain, and irritation in the face and mouth. The “prickle” of a soda is a form of mild “chemo-nociception”—a tiny, controlled “pain” signal that the brain interprets as refreshing. In e-liquids, we must find chemicals that can trigger this trigeminal “sting” without causing actual discomfort.
In the soft drink industry, CO2 is forced into water at high pressure (often 30-45 psi). In an e-liquid bottle, maintaining such pressure is impossible. Furthermore, even if we could carbonate a PG/VG base, the physics of vaping would defeat the effort:
Therefore, the “fizz” in a vape is a sensory illusion created through three chemical pillars: Acidity, Cooling, and Volatile “Lift.”

Carbon Dioxide Molecule Diagram
The most critical component of the “fizz” illusion is the use of organic acids. Because the brain associates the stimulation of sour-sensing cells with carbonation, we use a specific blend of acids to mimic the pH drop that occurs when CO2 dissolves in water.
Malic acid is the primary tool for “fizzy” vapes. It provides a sharp, immediate “tingle” on the edges of the tongue. Unlike other acids, Malic acid has a “lingering” sourness that mimics the way the carbonic acid sensation stays in the mouth after a sip of soda.
Citric acid is used for “brightness.” In beverage profiles like Lemon-Lime or Orange Soda, Citric acid provides the initial “zing.” However, Citric acid can be hard on coils (causing “gunking”), so it is often used in a 10% dilution in PG.
For “Grape Soda” or “Champagne” profiles, Tartaric acid is essential. It provides a “dryer” mouthfeel and a heavier “bite” that mimics the tannins and carbonation found in fermented or grape-based beverages.
The target for a “fizzy” e-liquid is usually a pH between 3.5 and 4.5. If the pH drops too low, the flavor becomes “harsh” rather than “fizzy.” If it is too high, the flavor tastes “flat.”
A lukewarm soda never tastes as fizzy as a cold one. This is because cold temperatures sensitize the TRPM8 receptors in the mouth, which in turn amplifies the trigeminal nerve’s response to the “fizz.” In vaping, we use “Coolada” or specialized cooling agents to simulate this.
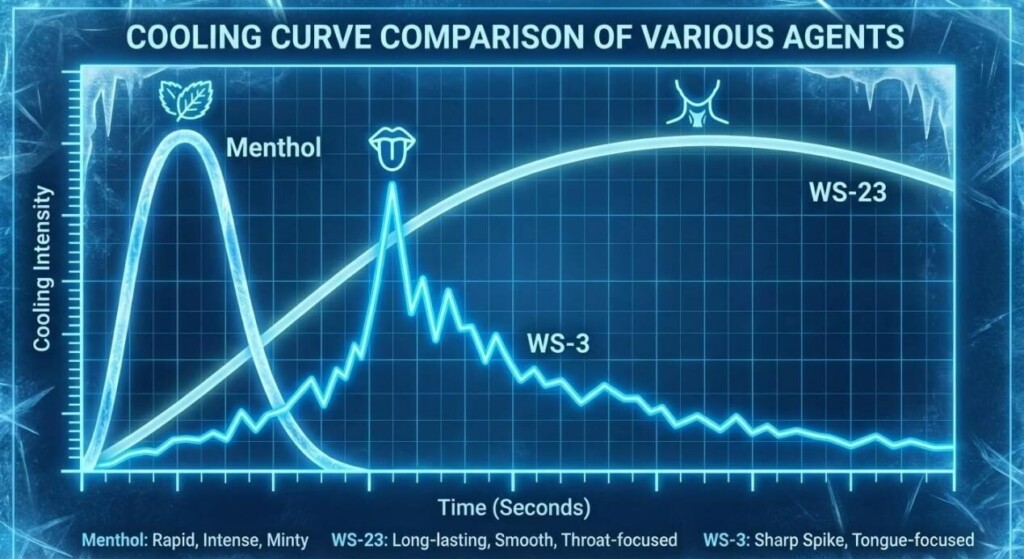
Cooling Agent Curve Comparison
WS-23 is the industry standard for “fizzy” vapes because it provides a clean, intense coldness with no minty aftertaste. By cooling the vapor, we create the environmental conditions required for the brain to accept the “fizz” illusion.
While WS-23 cools the throat, WS-3 focuses more on the tongue and roof of the mouth. A blend of WS-23 and WS-3 is often used to create a “full-mouth” coldness that mimics the experience of drinking an ice-cold carbonated beverage.
When you open a soda, the first thing you experience is an “aromatic burst”—the gas carrying volatile flavor molecules into your nose. To replicate this in a vape, we use high-volatility esters.
Ethyl Acetate is a common ester used to provide “lift.” It has a slightly fruity, “ethereal” scent and a very low boiling point. When added in minute quantities, it helps the other flavors “pop” the moment the vapor hits the palate, mimicking the way carbonation carries aromatics.
For citrus sodas, the “fizzy” sensation is enhanced by using “top-heavy” citrus notes. Citral provides a sharp, candy-like lemon bite, while Limonene provides the zesty, sparkling top note of an orange or lime.
In sp arkling wine profiles, this ester (often called “Strawberry Aldehyde,” though it is an ester) provides a “fizzy fruit” nuance that, when combined with Tartaric acid, creates a convincing “bubbly” sensation.
One of the most persistent “hacks” in e-liquid DIY circles is the addition of small amounts of Sodium Bicarbonate (Baking Soda) or Potassium Bicarbonate. The theory is that the heat of the coil will cause the bicarbonate to release CO2 gas directly into the vapor.
While this technically works on a molecular level, it presents significant manufacturing challenges:
As a professional manufacturer, we generally avoid bicarbonates in favor of Ethyl Maltol and Triacetin, which can modify the “mouthfeel” to be “lighter” and more “aerated” without destroying the hardware.
CITATION 2: The Flavor and Extract Manufacturers Association (FEMA) provides safety data and “GRAS” (Generally Recognized as Safe) lists for flavoring substances. Their research into esters and organic acids forms the basis for the safety profiles of the “fizz” components used in ENDS.
Source: FEMA GRAS Lists. Available at: https://www.femaflavor.org/gras
A “flat” vape often feels “syrupy” or “heavy” due to the high VG content. To make a flavor feel “fizzy,” we must “lighten” the mouthfeel.
Triacetin (Glycerin Triacetate) is a solvent and a mouthfeel modifier. It has a “thinning” effect on the perception of the vapor, making it feel less like a heavy cloud and more like a “sharp” mist. This “thinning” is crucial for beverage profiles.
While Sucralose is the standard sweetener for vapes, it can feel “heavy” and “sticky.” Many “fizzy” formulations use Erythritol. Erythritol has a lower sweetness level but provides a “cooling” sensation as it dissolves (the “heat of solution”). This adds a “crispness” to the finish of the vape that Sucralose cannot match.
Fizzy Flavor Mapping Infographic
To see these principles in action, let’s look at how we would build a “Fizzy Cola” flavor concentrate from the ground up.
When vaped, the user first feels the cold (WS-23), followed immediately by the acidic tingle (Malic Acid). As they exhale, the volatile esters (Ethyl Acetate) carry the cola aromatics through the retro-nasal passage, creating a “bubbly” sensory experience that mimics a glass of fresh cola.
Creating a “fizzy” flavor is one thing; making it shelf-stable is another.
Over time, high concentrations of Malic or Citric acid can cause “esterification” or “hydrolysis” of other flavor molecules. For example, a “Strawberry Soda” may lose its strawberry “brightness” over six months because the acid is slowly breaking down the strawberry esters.
CITATION 3: A study in the Journal of Agricultural and Food Chemistry notes that the stability of esters in acidic environments is a major concern for shelf-life, as the pH of the solution directly influences the rate of aromatic degradation.
Source: “Stability of Flavor Esters in Acidic Media,” ACS Publications. Available at: https://pubs.acs.org/journal/jafcau
Acidic “fizzy” vapes are notorious for shortening coil life. The combination of sweeteners (like Sucralose) and organic acids creates a “syrup” that caramelizes quickly on the heating element. To combat this, we recommend:
As with all flavoring components, safety is paramount. The vaping industry is under intense scrutiny from bodies like the FDA and TPD.
CITATION 4: The UK Medicines and Healthcare products Regulatory Agency (MHRA) and the Tobacco Products Directive (TPD) in Europe set strict limits on additives. Their guidelines emphasize that flavoring chemicals must not create a risk to human health in their heated form.
Source: “Guidance on E-cigarette notifications,” MHRA. Available at: https://www.gov.uk/guidance/e-cigarettes-regulations-for-consumer-products
The quest for the “Perfect Fizz” continues. We are currently exploring several new chemical frontiers:
While WS-23 targets the “cold” receptors, we are looking at molecules that specifically target the TRPA1 (transient receptor potential ankyrin 1) receptors. These are the receptors that respond to the “sting” of mustard or horseradish. In micro-doses, they could provide a “sparkle” that is even more realistic than Malic acid.
By encapsulating organic acids in a heat-sensitive lipid shell, we could potentially protect the coil from “gunking” while allowing the acid to release only when it reaches the necessary temperature for vaporization.
We are working on “low-density” flavor molecules that have a higher “vapor pressure,” allowing them to expand more rapidly upon inhalation, creating a “fluffy” or “bubbly” mouthfeel.
The “fizz” in a vape flavor is a testament to the power of modern flavor chemistry. It is a symphony of neurobiology, organic chemistry, and sensory psychology. We don’t need CO2 to create a “sparkle”; we only need to understand the triggers that the human brain uses to identify that sensation.
Through the precise application of Malic acid for the “tingle,” WS-23 for the “cold,” and volatile esters for the “lift,” we can transport a vaper to a refreshing glass of their favorite soda. As we continue to refine our “fizz” packages, we remain committed to the balance of intensity, stability, and—above all—safety.

Fizzy Concentrate Laboratory Testing
Are you ready to elevate your beverage flavor line with a truly authentic “fizzy” experience? Our technical team is ready to assist you in formulating the perfect “sparkle” for your brand.
| Contact Channel | Details |
| 🌐 Website: | www.cuiguai.com |
| 📧 Email: | info@cuiguai.com |
| ☎ Phone: | +86 0769 8838 0789 |
| 📱 WhatsApp: | +86 189 2926 7983 |
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy