Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Nov 3, 2025

E-liquid Stability Testing
In the world of e-liquid fragrance manufacturing, it’s not sufficient to develop a great aroma profile in the laboratory and assume it will deliver flawlessly at the point of use. A major determinant of success is how well the fragrance concentrate survives the rigours of shipping, warehousing and storage before being used in final product blends. Many flavour houses—or fragrance manufacturers supplying e-liquid aroma systems—underestimate the impact of logistics, environment and elapsed time on aroma performance.
This blog post is written for you—the professional manufacturer of fragrances for e-liquids—and it provides a technically-rich, authoritative, and well-structured examination of stability testing in the context of shipping and storage. We cover:
By the end of this article you will understand not only why stability testing is essential, but how to conduct it, what pitfalls to avoid and how to embed it as a competitive advantage in your business.
When you supply fragrance concentrates for e-liquids, your customer (the e-liquid producer) expects the aroma system to perform identically from its creation in your lab through it arriving at their filling line (sometimes thousands of kilometers away). If the concentrate has degraded during transit or while awaiting use, then the final product may deliver weaker aroma, off-notes, inconsistent performance or even consumer complaints. In effect you’re responsible for flavour integrity across the entire supply chain, not just in batch release.
Stability failures lead to re-work, batch rejects, additional testing, logistic delays, and potentially lost customers. In an industry where regulatory scrutiny, flavour specification documentation and repeatability matter, being able to certify your fragrance’s performance after shipping and storage gives you a competitive edge.
Fragrance concentrates for e-liquids often include volatile compounds, solvent systems (PG, VG, ethanol), cooling agents, sweeteners or aroma modifiers. Unlike many food flavour systems, e-liquid aroma concentrates are subject to further aerosolisation, coil heat, and interaction in the final formulation. If the concentrate has degraded (oxidation, hydrolysis, evaporation) before it reaches the client, the downstream performance may be compromised. A recent review of flavour retention found that volatile compound loss and matrix effects are major stability issues.
Hence, establishing that your fragrance can “survive shipping and storage” is not optional—it’s a mark of technical robustness and quality assurance.
Several physical, chemical and sensory degradation pathways can undermine fragrance performance in your concentrates. Understanding them helps you build a focused stability testing strategy.
Elevated temperatures accelerate chemical reactions (oxidation, hydrolysis), increase volatility of aroma compounds, promote container‐headspace interactions, and can drive loss of aroma intensity. A third-party review of transport/storage conditions for wines noted that higher transport/warehouse temperatures caused significant sensory deterioration and chemical changes.
For fragrance concentrates:
One of the major risks for aroma concentrates is the physical loss of volatile components over time—either by permeation through packaging or repeated opening/closing. Some flavour modules may lose key aroma compounds and thus under-deliver. The general concept of “shelf life” applies: “The length of time that a commodity may be stored before it degrades.”
During storage, flavouring molecules may undergo chemical transformations:
The integrity of the drum, the seal, the headspace, choice of material (HDPE vs amber glass vs stainless IBC), barrier properties all influence stability. Improper storage conditions (e.g., open drums, exposure to sunlight) can compromise quality. One article on chemical storage of flavouring chemicals emphasised that flammable/volatile flavouring chemicals require controlled storage buildings with ventilation, temperature control and segregation.
While less chemical and more logistical, rough handling, vibration, jostling may cause leaks, container deformation, micro-headspace changes, or penetration of contaminants. Also, shipping cold → hot transition may cause condensation problems.
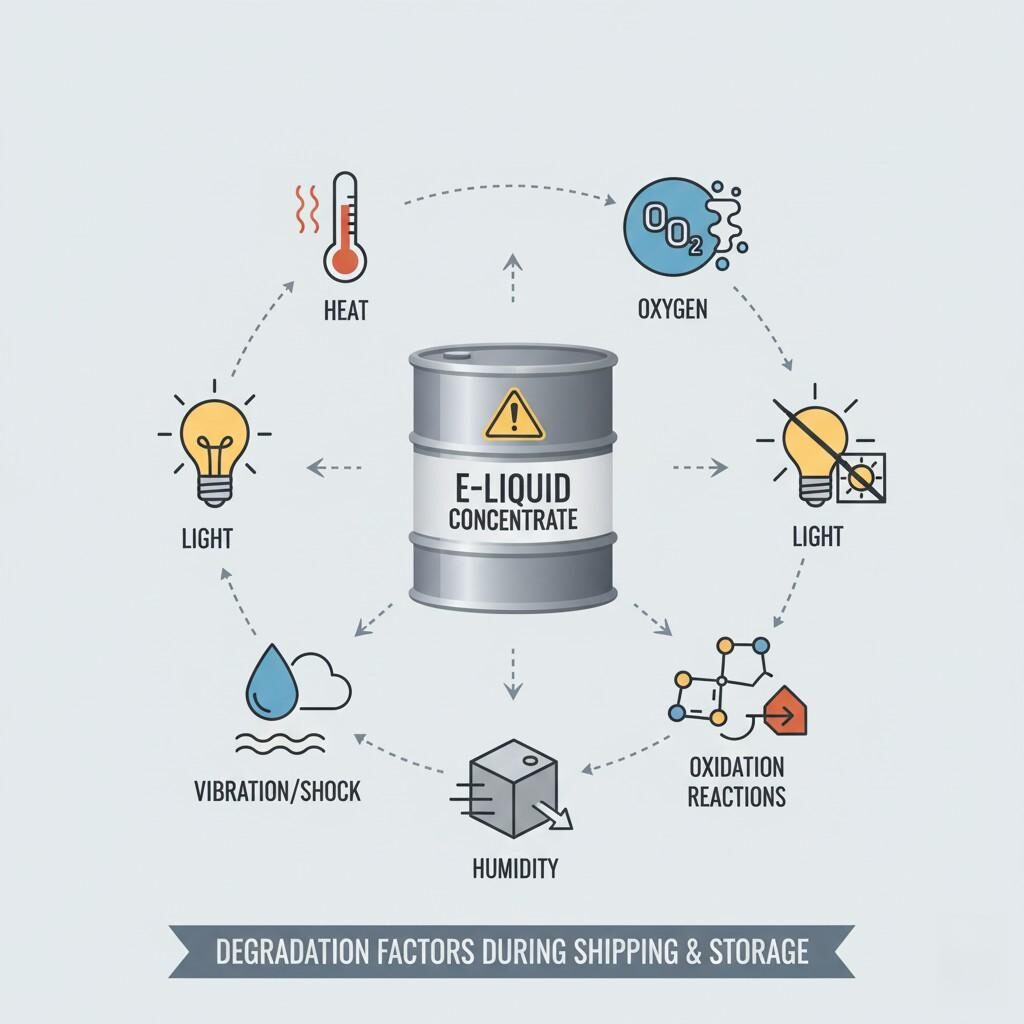
E-liquid Degradation Factors Infographic
Given the mechanisms above, it is vital to build a purposeful testing protocol that mimics or accelerates real-life shipping and storage conditions and gives you confidence in your concentrate’s performance.
Select conditions based on your real logistics profile:
Define durations:
Sample units: Use representative production lot drums or IBCs, include control samples kept at ideal conditions. Ensure replicates.
Endpoints to measure:
Establish your quality acceptance criteria upfront:
Keep thorough records: each stability test batch lot number, packaging, conditions, analytical results, sensory panel results, chart of key marker degradation, trend lines. These documents increase your credibility and help when clients audit your fragrance manufacturing process.
Beyond laboratory tests, consider field shipments: send real drums via typical logistic chain (e.g., intercontinental shipping, humidity variation, temperature extremes) with data loggers. On arrival, sample the concentrate and test against your criteria. This gives real-world validation and client confidence.

Aroma Concentrate Stability Testing Lab
Once your stability test protocol yields data, you need to interpret results and determine whether the fragrance concentrate is fit for purpose, or if reformulation/packaging changes are needed.
When performance fails, apply root-cause analysis:
Using your stability data (marker degradation rate, sensory impact, packaging integrity), you can define a recommended use-by period for your fragrance concentrates (e.g., “Best used within 12 months of dispatch if stored at ≤25 °C”). Use these labels and guidelines to protect your brand and to be transparent with clients.
Provide your clients (the e-liquid manufacturers) with:
This level of transparency reinforces your credibility and positions you as a technical partner, not just a concentrate supplier.
Here we discuss practical steps you, as a fragrance manufacturer, can implement to enhance shipping and storage stability of your e-liquid fragrance concentrates.
For you as a manufacturer of fragrances for e-liquids, stability testing for shipping and storage must become part of your process, not an afterthought.
– During flavour module development, include stability screening (thermal, light, humidity) of candidate aroma systems.
– Use accelerated screening to filter out unstable aroma compounds before full formulation.
– Document key performance metrics: aroma marker concentration, volatility, headspace loss, container compatibility.
– Create a stability design space: ranges of temperature, time, container conditions your module can tolerate.
– For each flavour module provide:
– Work with your logistics partners to understand transit routes, temperature/humidity profiles, shipment duration.
– Include shipping simulation in your validation: e.g., send drums on typical route and include temp/humidity data logger.
– Include monitoring, corrective action thresholds and escalation if shipping conditions exceed spec.
– Maintain records of customer feedback relating to aroma performance post-shipment/storage.
– Review stability data periodically, refine modules if degradation trends emerge.
– Use trending analysis of stability test results, shipping logs, customer batch returns to drive improvement.
– Provide training internally (R&D, quality, logistics) on the importance of shipping/storage stability.
When you can demonstrate that your fragrance concentrates are validated for shipping and storage under real-world conditions you gain a competitive advantage:
In summary: yes—the question “Can your flavouring survive shipping and storage?” must be answered affirmatively if you aim for premium performance, customer trust and operational excellence. The key is to build a rigorous stability testing programme, simulate real-world shipping/storage conditions, and embed packaging, formulation and logistics controls into your fragrance manufacturing process.
By doing so you:
To recap: the major degradation mechanisms—temperature, humidity/oxygen/light, container headspace, evaporation and chemical transformation—are controllable. With the right test protocols, packaging, formulation and logistics design you ensure that your fragrance concentrates arrive at the client’s line ready to perform.
We encourage your R&D, quality and logistics teams to adopt the described strategy and start today. The sooner you validate your modules for shipping/storage, the more confident your clients will be—and the stronger your brand differentiation will become.

Fragrance Warehouse Global Logistics
If you’re ready to validate your fragrance concentrates for shipping and storage—and provide your clients with documentation of stability under real-world conditions—let’s collaborate. Contact us today for a technical exchange and request a free sample kit of our stability-qualified aroma modules. Together we’ll build fragrance solutions that don’t just smell great—but arrive and perform reliably.
🌐 Website:[www.cuiguai.com]
💬 Whatsapp:[+86 189 2926 7983]
📩 Email:[info@cuiguai.com]
📞 Phone: [+86 0769 8838 0789]
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy