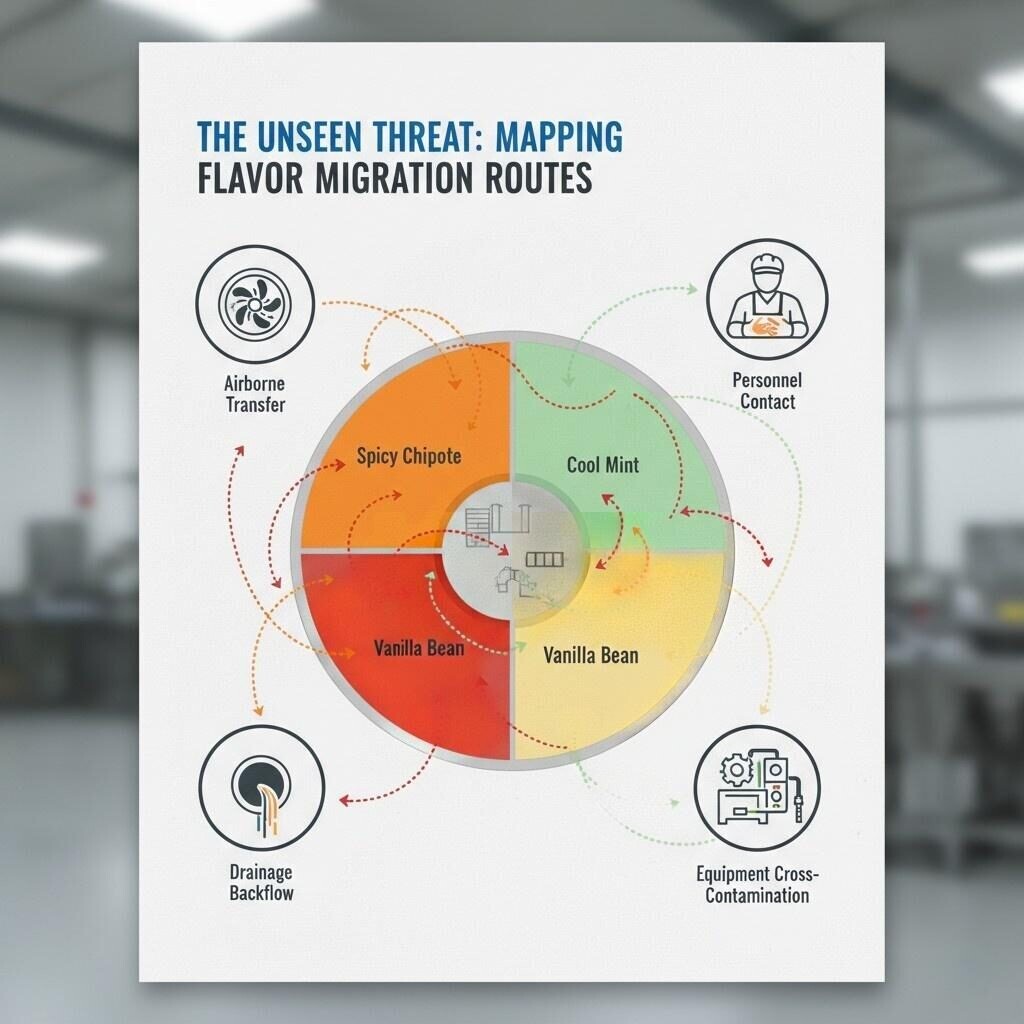
Flavor Migration Routes
The food, beverage, and vape industries have witnessed a significant surge in the adoption of shared manufacturing facilities and co-packing arrangements. This model offers compelling benefits: reduced capital expenditure, accelerated time-to-market, and access to specialized equipment and expertise. However, beneath the surface of these operational efficiencies lies a critical, and often underestimated, technical challenge: flavor migration.
Flavor migration, or more accurately, cross-contamination, is the silent threat that can undermine a product’s integrity and a brand’s reputation. It occurs when a trace amount of one flavor is unintentionally transferred to a subsequent batch, creating an undesirable off-note or completely altering the intended sensory profile. For a brand that has invested heavily in creating a unique flavor identity, the presence of an unwanted note—be it a hint of strawberry in a coffee e-liquid or a trace of mint in a dessert line—is not just a quality control issue; it is a fundamental breach of consumer trust.
Mitigating this risk requires a comprehensive, science-based strategy that extends far beyond standard cleaning protocols. It is a multi-faceted approach that combines advanced engineering controls, a rigorous analytical validation program, and a culture of uncompromising operational discipline. This comprehensive technical guide will serve as a definitive blueprint for understanding the mechanisms of flavor migration and implementing the necessary measures to ensure product purity and safeguard brand integrity in a shared manufacturing environment.
To effectively combat flavor migration, we must first understand its invisible, insidious nature. The contamination does not happen by accident; it occurs through specific, scientifically predictable vectors. The challenge lies in the fact that flavor compounds are often volatile and can exist at concentrations so low they are undetectable by the human nose but are still potent enough to affect taste.
The most common and hardest-to-control vector of flavor migration is through the air. Flavor molecules are essentially volatile organic compounds (VOCs).
Flavor compounds, especially those with high viscosity like certain extracts or those with strong lipid solubility, can cling to surfaces.
Personnel moving between different production areas are a significant vector for flavor migration.
Many cleaning protocols are designed to remove visible residues or sanitize against microbes, not to eliminate sub-micron flavor compounds. Standard detergents and rinses are often insufficient for removing lipophilic (oil-based) flavor molecules that bind to surfaces. This highlights the need for specialized, scientifically validated cleaning procedures.
In the fight against flavor migration, you cannot manage what you cannot measure. A simple sniff test or a visual inspection is fundamentally inadequate. The only way to ensure a surface is truly clean is through rigorous analytical validation.
A flavor can be perceived by the human nose at concentrations as low as parts-per-trillion (ppt). Standard quality control methods, such as a sensory panel test of a production batch, will only catch a problem after a product has been made. To prevent the problem, you must be able to detect flavor residues on a surface at the parts-per-billion (ppb) level, a task that requires advanced analytical equipment.
We use a suite of powerful analytical tools to detect and quantify trace flavor residues on equipment surfaces.
Our cleaning validation protocol is a systematic, data-driven process that provides an objective, unassailable guarantee of cleanliness.
Step 4: Acceptance Criteria: The results are compared against a pre-established Acceptance Criteria. If the residue level is below the pre-defined ppb limit, the equipment is released for the next batch. If not, the cleaning process is repeated and re-validated.
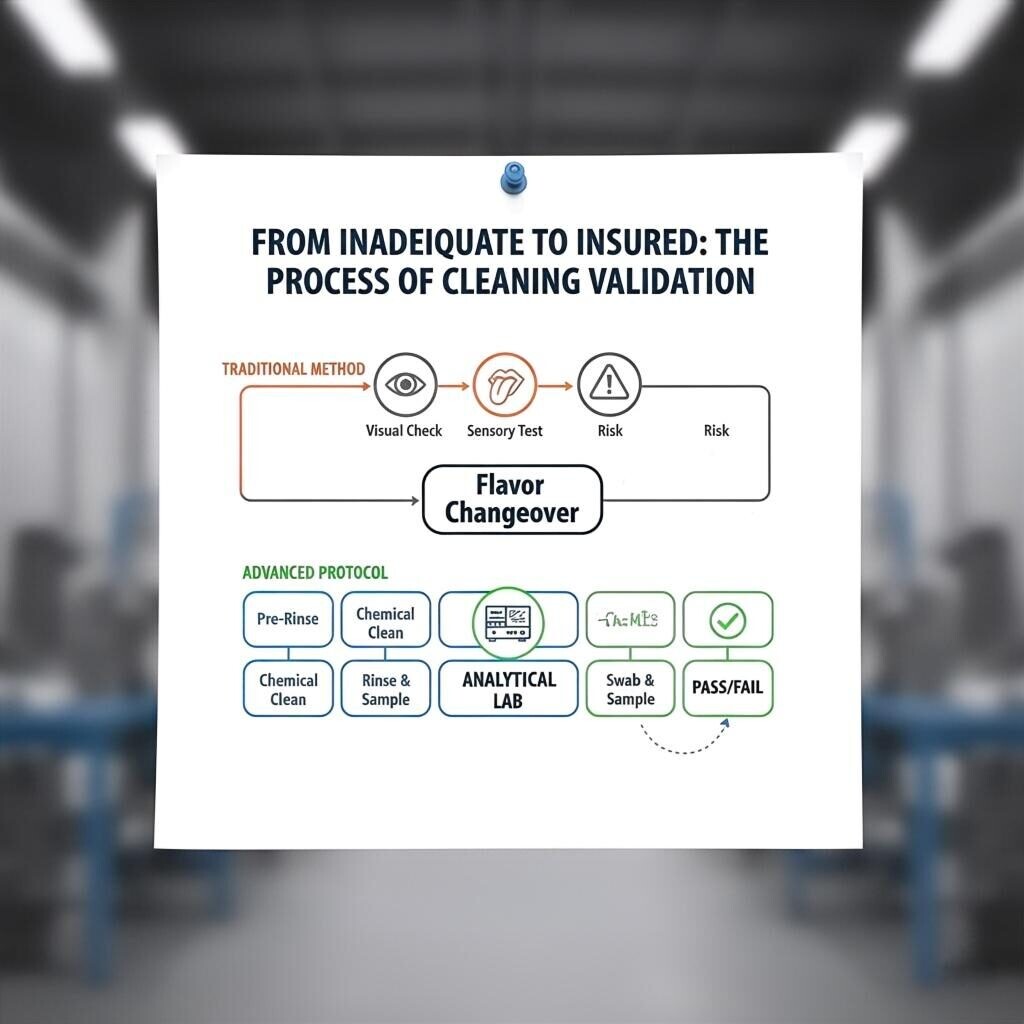
Cleaning Validation Flowchart
While operational procedures are critical, they are only as effective as the environment in which they are performed. A facility’s engineering and design are a primary line of defense against flavor migration.
Proper Heating, Ventilation, and Air Conditioning (HVAC) systems are a manufacturer’s most powerful tool against airborne flavor migration.
The best way to prevent cross-contamination is to eliminate the possibility of it happening in the first place.
The materials used in a facility’s construction and equipment are not a trivial detail.
Even the most advanced facility and equipment are useless without a team that understands and adheres to a strict protocol of operational excellence.
Every flavor changeover requires a pre-defined, written Master Cleaning Plan. This document is a critical part of our Current Good Manufacturing Practices (cGMP).
A highly trained team is a primary line of defense.
The Flavor and Extract Manufacturers Association (FEMA)‘s safety and quality guidelines place a significant emphasis on personnel training and a culture of food safety as a cornerstone of product integrity (Reference 3: FEMA, “Good Manufacturing Practices for Flavors,” 2024).
For a shared manufacturing partner, a robust flavor migration protocol is not just an expense; it is a strategic investment that provides a significant competitive advantage.
A proactive co-packer will conduct a formal risk assessment for every flavor. This involves identifying potential sources of contamination and designing specific mitigation strategies for each one. This level of diligence provides a level of client confidence that generic co-packers cannot match.
A single case of flavor migration can lead to a product recall, which is not only costly but also catastrophic for a brand’s reputation. Legal liabilities and consumer lawsuits related to mislabeled or contaminated products are a serious risk. The US Food and Drug Administration (FDA)‘s Food Safety and Modernization Act (FSMA) holds co-packers and brands equally responsible for product safety, making a robust cleaning validation protocol a legal necessity (Reference 4: FDA, FSMA Guidelines on Co-packer Responsibilities, 2023).
In an industry where brands are highly sensitive to their product’s integrity, a co-packer’s ability to demonstrate a commitment to flavor purity is a powerful selling point. It builds a foundation of trust that leads to long-term partnerships and a premium position in the market. A robust flavor migration protocol is a guarantee of quality that elevates a co-packer from a simple service provider to a strategic partner.

The Promise of Purity
In the complex ecosystem of shared manufacturing, flavor migration is the ultimate test of a facility’s commitment to quality. It is a silent, but serious, threat that can undermine the value of a brand and the trust of its consumers.
Mitigating this risk is not a trivial task; it is a complex, technical imperative that requires a systematic, scientific, and data-driven approach. It is an investment in advanced analytical tools, intelligent facility design, and a culture of uncompromising operational discipline. By understanding the mechanisms of contamination and implementing the strategies outlined in this guide, a brand can safeguard its products, a co-packer can build an unassailable reputation for quality, and the entire industry can move toward a new standard of purity and trust. In the final analysis, the pursuit of absolute flavor integrity is not an option; it is the foundation of lasting success.
Keywords: vape flavor cross-contamination, shared facility flavor risk
Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Sep 11, 2025
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy