Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.

E-liquid Lab & Compliance
In the rapidly evolving e-liquid industry, safety, transparency, and compliance have become as crucial as creativity and sensory appeal.
Since the introduction of the Tobacco Products Directive (TPD) by the European Union, manufacturers of e-liquids and vape flavorings are required to meet strict regulatory standards governing product formulation, labeling, and market authorization.
For professional flavor manufacturers and vape brands, understanding TPD compliance is no longer optional — it is a technical and legal necessity that directly affects market access, consumer trust, and long-term brand sustainability.
This guide explores, in depth, how to formulate e-liquid flavorings that fully comply with TPD regulations — from ingredient selection and toxicological screening to submission protocols and labeling requirements.
We will also discuss practical formulation tips and scientific best practices that ensure flavor quality, safety, and regulatory conformity.
The Tobacco Products Directive (2014/40/EU), implemented in May 2016, established a unified regulatory framework across all EU member states for the manufacturing, presentation, and sale of tobacco and related products — including e-cigarettes and refill liquids.
According to Article 20 of the Directive, e-liquids containing nicotine must comply with specific composition, notification, and packaging requirements [¹].
Even though flavorings are not always classified as “tobacco” components, any ingredient used in a product intended for inhalation must meet the same purity and toxicological standards.
For e-liquid flavor developers, this means each aroma compound — from fruit esters to sweeteners and cooling agents — must be evaluated, documented, and disclosed in a detailed regulatory dossier.
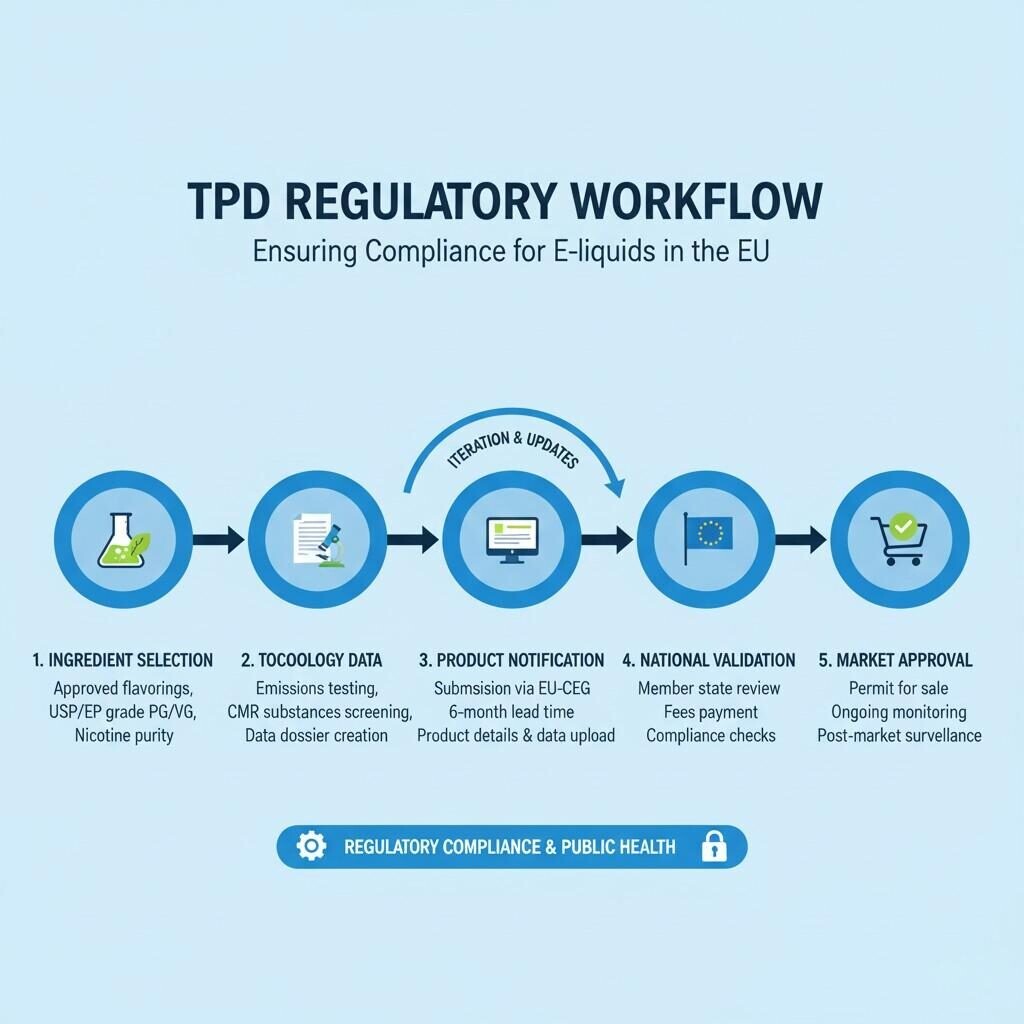
TPD Regulatory Workflow
The TPD’s requirements extend not only to e-liquid brands but also to flavor suppliers, as they provide the raw materials used in final nicotine-containing products.
All substances used in e-liquid flavorings must:
Flavor compounds should also be evaluated for thermal decomposition risks to ensure that no harmful byproducts (formaldehyde, acetaldehyde, etc.) form during heating.
Manufacturers must provide:
These documents are critical for TPD notification submissions and must be reviewed by qualified toxicologists.
Every e-liquid product containing nicotine must be notified to the EU Common Entry Gate (EU-CEG) portal at least 6 months before marketing.
This process requires detailed disclosure of:
Even non-nicotine e-liquids are now subject to similar national-level requirements in many EU countries (e.g., France, Germany, and Italy).
The TPD bans or restricts any additive that:
Commonly prohibited or restricted compounds include:
| Compound | Reason for Restriction | TPD Compliance Status |
| Diacetyl (2,3-butanedione) | Respiratory risk (bronchiolitis obliterans) | Prohibited |
| Acetoin | Potential precursor to diacetyl formation | Restricted |
| Acetyl propionyl | Similar toxicity to diacetyl | Prohibited |
| Cinnamaldehyde | Cytotoxicity under vaporization | Restricted |
| Coumarin | Toxicological concerns (liver toxicity) | Prohibited |
| Essential oils (unrefined) | May contain allergens or reactive terpenes | Restricted |
According to the European Chemicals Agency (ECHA), all substances must be screened under the CLP Regulation (EC No. 1272/2008) for hazard classification before inclusion in e-liquid flavor formulations [²].
Formulating compliant e-liquid flavorings is not just about removing restricted compounds — it involves technical balance between sensory design and chemical stability.
Common flavor carriers like propylene glycol (PG) and triacetin must meet:
Solvent ratio affects flavor volatility, diffusion rate, and coil performance — crucial parameters in consumer safety and product consistency.
Natural extracts can enhance authenticity but often contain trace allergens or unstable terpenes.
Synthetic analogs offer purity, consistency, and control, making them preferred for regulatory compliance.
A hybrid approach — combining natural-identical molecules with controlled synthetics — often achieves both TPD safety and flavor fidelity.
Every compound must be tested for thermal stability under vaporization conditions (typically 180–250°C).
Compounds that decompose into aldehydes, ketones, or acids under heat should be replaced or reformulated.
Advanced GC–MS and TGA analysis can identify potential breakdown pathways, ensuring a flavor remains inert and safe during vaping.
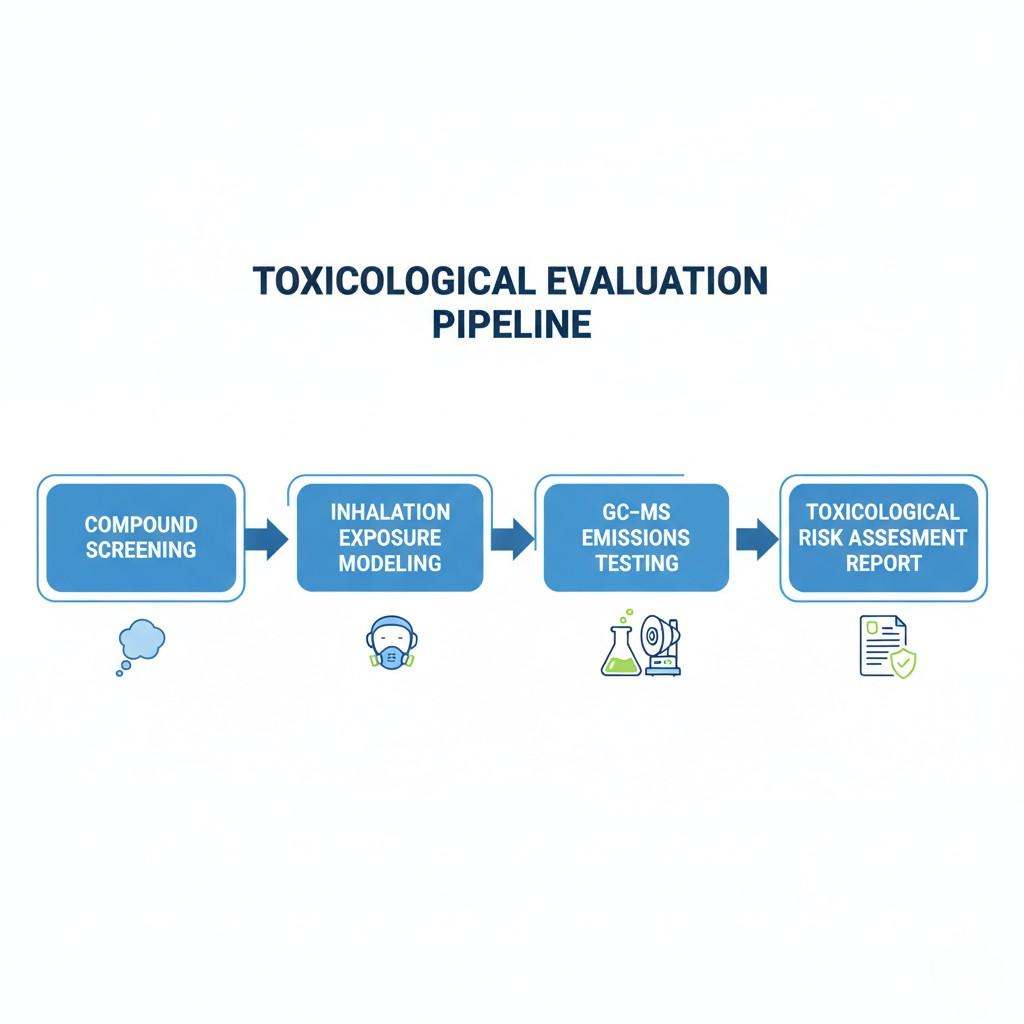
Toxicological Evaluation Flowchart
The TPD explicitly requires manufacturers to provide information on toxicological data of each ingredient, particularly in the context of inhalation exposure.
This distinguishes e-liquid flavor formulation from food or cosmetic flavoring.
A professional evaluation includes:
Industry guidelines from the Flavor and Extract Manufacturers Association (FEMA) and European Food Safety Authority (EFSA) provide reference exposure limits for common aroma compounds [³].
E-liquid flavor formulators must ensure ingredient usage levels remain well below inhalation-based toxicity thresholds, even under concentrated conditions.
A complete TPD submission dossier includes the following technical files:
These documents are uploaded via the EU Common Entry Gate (EU-CEG) platform, where each product receives a unique submission ID recognized by all EU Member States.
Submission fees and procedures vary slightly by country but follow harmonized EU guidelines, as outlined by the European Commission’s Health and Food Safety Directorate (DG SANTE) [⁴].
TPD compliance extends beyond paperwork — it requires systematic control at every production stage.
E-liquid flavor facilities should operate under:
Batch samples should be retained for 2 years minimum, ensuring traceability for audits or incident investigations.
Even trace allergens like limonene, linalool, or citral must be declared and limited.
All raw materials must be screened for:
Routine GC–MS purity testing ensures flavor formulations remain compliant and safe for long-term inhalation.
Each production lot should have:
Digital compliance management software can automate reporting for EU-CEG submissions and ISO 9001 documentation.

E-Liquid Label Example
TPD-compliant e-liquid labels must include the following elements:
All ingredients, including flavorings, must be listed by descending weight and include relevant CAS numbers if requested.
All e-liquid containers must be:
Under Article 20, no claims suggesting health, vitality, or reduced harm may be made.
Flavor names and imagery should avoid references to foods, beverages, or candies appealing to minors.
| Mistake | Consequence | Preventive Action |
| Using food-grade but not inhalation-tested flavors | Regulatory rejection or health risk | Use only TPD-screened ingredients |
| Missing toxicology documentation | EU-CEG submission failure | Partner with accredited toxicology consultants |
| Non-conforming labeling (font size, warnings) | Product recall or fines | Follow Article 20 label templates |
| Inconsistent batch records | Loss of compliance traceability | Implement digital GMP tracking |
| Ignoring national variations (e.g., France ANSES) | Market entry delays | Review local implementation guides |
Compliance is not a one-time task but an ongoing process of monitoring, documentation, and re-evaluation as regulatory science evolves.
As vaping technology advances, future revisions to the TPD are expected to tighten ingredient controls, expand non-nicotine coverage, and increase toxicological transparency.
Industry experts anticipate:
Manufacturers that proactively align with scientific risk assessment methods, transparent labeling, and sustainable ingredient sourcing will be best positioned for long-term success in both EU and global markets.
TPD compliance is not a bureaucratic burden — it’s a framework for safety, quality, and consumer confidence.
By integrating compliance from the earliest stages of flavor formulation, manufacturers can differentiate themselves through trust and technical excellence.
At CUIGUAI Flavoring, we specialize in TPD-compliant e-liquid flavor systems — rigorously tested for purity, stability, and sensory performance.
Our flavors are backed by GC–MS analysis, toxicological documentation, and ISO-certified quality systems, ensuring full regulatory compatibility across European and global markets.
Ready to develop TPD-compliant e-liquid flavors for your next product line?
Contact our technical experts for formulation support, compliance consulting, or free flavor samples tailored to your regulatory needs.
👉 Request Technical Consultation or Free Sample
📩 [info@cuiguai.com]
📞 [+86 189 2926 7983]
🌐 Explore more at 【www.cuiguai.com】
[¹] European Parliament and Council. Directive 2014/40/EU on the manufacture, presentation and sale of tobacco and related products. (EUR-Lex, 2016).
[²] European Chemicals Agency (ECHA). CLP Regulation (EC No. 1272/2008). https://echa.europa.eu/clp-regulation
[³] European Food Safety Authority (EFSA). Scientific Opinion on the evaluation of flavoring substances. EFSA Journal, 2022.
[⁴] European Commission DG SANTE. EU Common Entry Gate (EU-CEG) User Guide. 2023.
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy